Abstract
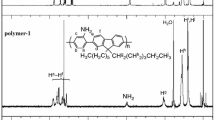
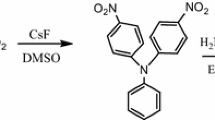
Phenylethynylene-substituted poly(triphenylamine vinylene) were synthesised in two steps by Wittig polycondensation reaction between 4,4'-diformyl-triphenylamine and bis(4-formyl phenyl)-N,N'-iodo-phenylamine in the presence of a phosphonium salt. The iodine-substituted poly(triphenylamine vinylene) were subsequently subjected to coupling reactions with phenylacetylene obtaining conjugated new structures and properties. The structures were confirmed by C13 and 1H nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FTIR) spectroscopy. The attachment of phenylethynyl substituent to the backbone of the polymers influences the optical and electrochemical properties which were analysed by ultraviolet-visible (UV-Vis) and fluorescence spectroscopy. Having triphenylamine units along the backbone, the obtained polymers exhibit electrochemical activity and their redox characteristics were investigated by running cyclic voltammetry for polymer films deposited on working electrode surface. The electrochemical data were used to estimate their highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels and the band gap energy values (Eg). The polymers films change their colour while the potential is swept on the positive anodic domain, and this may be due to various oxidation states that undergo the polymers. The in situ UV-Vis vs. applied potential spectra were also recorded.

Similar content being viewed by others
References
B. Minaev, G. Baryshnikov, and H. Agren, Phys. Chem. Chem. Phys., 16, 1719 (2014).
M. Mondragón, J. U. Balderas, L. G. Jimenez, M. E. Sánchez-Espíndola, and C. Falcony, Org. Electron., 15, 2993 (2014).
J. M. Hales, S. Barlow, H. Kim, S. Mukhopadhyay, J. L. Brédas, J. W. Perry, and S. R. Marder, Chem. Mater., 26, 549 (2014).
M. M. Murray, A. B. Holmes, Semiconducting Polymers: Chemistry, Physics and Engineering, G. Hadziinoannou and P. F. van Hutten, Eds., Wiley, VCH Weiheim, 2000.
S. K. Yook and Y. Y. Lee, Synth. Met., 162, 1594 (2012).
J. A. Mikroyannidis, M. M. Stylianakis, P. Suresh, and G. D. Sharma, Sol. Energy Mater. Sol. Cells, 93, 1792 (2009).
J. Kwak, W. Z. Bae, M. Zorn, H. Woo, H. Yoon, J. Lim, S. W. Kang, S. Weber, H. J. Butt, R. Zentel, S. Lee, K. Char, and C. Lee, Adv. Mater., 21, 5022 (2009).
M. Behl, E. Hattemer, M. Brehmer, and R. Zentel, Macromol. Chem. Phys., 203, 503 (2002).
B. S. Kim, S. H. Yoo, O. Dongkenn, C. S. Wook, C. D. Soo, C. E. Lee, and Y. I. Yin, Synth. Met., 145, 229 (2004).
H. J. Snaith, G. L. Whiting, B. Sun, N. C. Greenham, W. T. S. Huck, and R. H. Friend, Nano Lett., 5, 1653 (2005).
Y. J. Shirota, Mater. Chem., 15, 75 (2005).
M. Sommer, S. M. Lindner, and M. Thelakkat, Adv. Funct. Mater., 17, 1493 (2007).
K. Y. Law, Chem. Rev., 93, 449 (1993).
R. H. Baughman, J. L. Bredas, R. R. Chance, R. L. Elsenbaumer, and L. W. Shacklette, Chem. Rev., 82, 209 (1982).
T. Yamamoto, M. Takagi, K. Kizu, T. Maruyama, K. Kubota, H. Kanbara, T. Kurihara, and T. Kaino, J. Chem. Soc. Chem. Commun., 9, 797 (1993).
A. P. Davey, S. Elliott, O. Óconnor, and W. Blau, J. Chem. Soc. Chem. Commun., 14, 1433 (1995).
K. P. Akshaya, P. M. Sandra, K. Amit, S. Ritu, N. Modeeparamil, and P. Manorajan, J. Polym. Sci., Part A: Polym. Chem., 49, 832 (2011).
T. Ivan, L. Vacareanu, and M. Grigoras, Int. J. Polym. Mater., 62, 270 (2013).
Y.-H. Kim, J.-C. Park, H.-J. Kang, J.-W. Park, H.-S. Kim, J. H. Kim, and S.-K. Kwon, Macromol. Res., 13, 403 (2005).
T. Ivan, L. Vacareanu, and M. Grigoras, Macromol. Res., 21, 1059 (2013).
P. Strohriegl and J. V. Grazulevicius, Adv. Mater., 14, 1439 (2002).
C. Ego, A.C. Grimsdale, F. Uckert, G. Yu, G. Srdanov, and K. Müllen, Adv. Mater., 14, 809 (2002).
F. I. Wu, P. I. Shih, C. F Shu, Y. L. Tung, and Y. Chi, Macromolecules, 38, 9028 (2005).
F. S. Linang, Y. J. Pu, T. Kurata, J. Kido, and H. Nishide, Polymer, 46, 3767 (2005).
M. Grigoras and L. Stafie, Des. Monomers Polym., 12, 177 (2009).
Z. Xia, J. He, P. Peng, Y. Zhou, Y. Li, and W. Tian, Tetrahedron Lett., 48, 5877 (2007).
H. L. Jeong, S. Jiwon, J. Hwang, S.Y. Park, C. Haeyoung, and C. Myoungsik, Chem. Mater., 16, 456 (2004).
G. Lai, R. X. Bu, J. Santos, and E. A. Mintz, Synlett, 1997, 1275 (1997).
X. Haijian, H. Jiating, X. Bin, W. Shanpeng, L. Yaowen, and T. Wenjing, Tetrahedron, 64, 5736 (2008).
L. Vacareanu and M. Grigoras, J. Appl. Electrochem., 40, 1969 (2010).
T. Mallegol, S. Gmouth, A. A. Meziane, D. Blanchard, and O. Mongin, Synthesis, 11, 1771 (2005).
T. Ivan, L. Vacareanu, and M. Grigoras, Des. Monomers Polym., 17, 156 (2013).
Y. J., Wang, H. S. Sheu, and C. K. Lai, Tetrahedron, 63, 1695 (2007).
Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu, and A. Heeger, Synth. Met., 99, 243 (1999).
Z. A. Tan, E. Zhou, Y. Yang, Y. He, C. Yang, and Y. Li, Eur. Polym. J., 43, 855 (2007).
T. Lana-Villarreal, J. M. Campin, N. Guijarro, and R. Gómez, Phys. Chem. Chem. Phys., 13, 4013 (2011).
O. Yurchenko, D. Freytag, L. Borg, R. Zentel, J. Heinze, and S. Lidwings, J. Phys. Chem. B, 116, 30 (2012).
J. F. Ambrose, L. L. Carpenter, and R. F. Nelson, Electrochem. Soc., 122, 876 (1975).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vacareanu, L., Virag (Ivan), T. & Grigoras, M. Phenylethynylene-substituted poly(triphenylamine vinylene): Post-modification synthesis and (spectro)electrochemical properties. Macromol. Res. 24, 249–260 (2016). https://doi.org/10.1007/s13233-016-4033-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-016-4033-5