Abstract
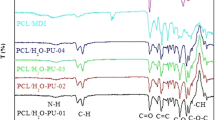
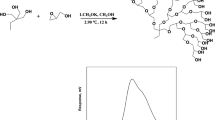
A series of novel biodegradable blocked polyurethane crosslinkers (BPUCs) were synthesized from the reaction of toluene 2,4-diisocyanate (TDI), isophorone diisocyanate (IPDI), polycaprolactone (PCL), 1,1,1-trimethylolpropane (TMP), and methyl ethyl ketoxime (MEKO). Synthesis of the accurate kind of BPUCs was confirmed by Fourier transform infrared spectroscopy (FTIR), proton nuclear magnetic resonance spectroscopy (1H NMR), and gel permeation chromatography (GPC). Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were used to determine the deblocking temperatures of the BPUCs. The thermal analysis revealed that both TDI- and IPDI-based BPUC had the proper initial deblocking temperature (<160 °C) and the maximum deblocking temperature (<200 °C) for practical applications. Compared to IPDI-based BPUC, the TDI-based BPUC had a lower thermal dissociation temperature and a faster deblocking rate. Hydroxyl polyurethane (HPU) was introduced to study the crosslinking effect of prepared BPUCs. The reaction proceeded at various deblocking temperatures in different curing times. It was noticed that the elastic modulus and tensile strength of the HPU sample increased whereas elongation at break decreased with the addition of BPUC in comparison with pure HPU, which suggested better interfacial adhesion due to the strong chemical reaction between the released NCO groups from BPUC and hydroxyl groups from HPU. In addition, improvement on water resistance of the BPUC modified HPU samples compared to pure HPU samples also demonstrated the good crosslinking effect of prepared BPUCs.

Similar content being viewed by others
References
A. S. Nasar, A. Raghavan, and V. S. Kumar, J. Macromol. Sci. Pure Appl. Chem., 42, 309 (2005).
T. K. Kim, B. K. Kim, S. Y. Lee, Y. L. Cho, M. S. Kim, and H. M. Jeong, Macromol. Res., 18, 177 (2010).
J. T. Choi, D. H. Kim, K. S. Ryu, H. Lee, H. M. Jeong, C. M. Shin, J. H. Kim, and B. K. Kim, Macromol. Res., 19, 809 (2011).
H. Yeganeh, M. M. Lakouraj, and S. Jamshidi, Eur. Polym. J., 41, 2370 (2005).
F. A. Puchkov and V. S. Turenko, Russ. J. Appl. Chem., 78, 1526 (2005).
M. Awkal, A. Jonquieres, R. Clement, and P. Lochon, Eur. Polym. J., 42, 1313 (2006).
S. Dutta and N. Karak, Polym. Int., 55, 49 (2006).
H. Yeganeh, S. Jamshidi, and P. H. Talemi, Eur. Polym. J., 42, 1743 (2006).
D. A. Wicks and Z. W. Wicks, Jr., Prog. Org. Coat., 36, 148 (1999).
D. A. Wicks and Z. W. Wicks, Jr., Prog. Org. Coat., 41, 1 (2001).
G. Oertel, Polyurethanes, Carl Hanser Verlag, Munich, 1993.
T. Xavier, B. Didier, and T. Lan, Eur. Polym. J., 36, 1745 (2000).
F. Schmitt, A. Wenning, and J. V. Weiss, Prog. Org. Coat., 34, 227 (1997).
D. A. Wicks and P. E. Yeske, Prog. Org. Coat., 30, 265 (1997).
G. Sankar and A. S. Nasar, Eur. Polym. J., 45, 911 (2009).
L. G. Yin, Y. L. Liu, Z. Ke, and J. H. Yin, Eur. Polym. J., 45, 191 (2009).
S. Subramani, J. M. Lee, and J. H. Kim, Macromol. Res., 13, 418 (2005).
E. M. Choi, K. S. Shin, and T. S. Hwang, Macromol. Res., 18, 577 (2010).
H. Kothandaraman and A. S. Nasar, Polymer, 34, 610 (1993).
S. Subramani, I. W. Cheong, and J. H. Kim, Prog. Org. Coat., 51, 329 (2004).
T. F. Shen, D. W. Zhou, L. Y. Liang, J. Zheng,Y. X. Lan, and M. G. Lu, J. Appl. Polym. Sci., 122, 748 (2011).
I. Ahmad, J. H. Zaidi, R. Hussain, and A. Munir, Polym. Int., 56, 1521 (2007).
S. Subramani, A. S. Nasar, T. P. Gnanarajan, N. P. Lyer, and G. Radhakrishnan, Polym. Int., 49, 546 (2000).
J. M. Lee, S. Subramani, Y. S. Lee, and J. H. Kim, Macromol. Res., 13, 427 (2005).
A. Karchin, F. I. Simonovsky, B. D. Ratner, and J. E. Sanders, Acta Biomater., 7, 3277 (2011).
X. K. Sun, H. Gao, G. L. Wu, Y. N. Wang, Y. G. Fan, and J. B. Ma, Int. J. Pharm., 412, 52 (2011).
J. S. You and S. T. Noh, Macromol. Res., 18, 1081 (2010).
B. J. Park, B. J. Kwon, J. K. Kang, M. H. Lee, I. Han, J. K. Kim, and J. C. Park, Macromol. Res., 19, 537 (2011).
J. L. Ryszkowska, M. Auguscik, A. Sheikh, and A. R. Boccaccini, Compos. Sci. Technol., 70, 1894 (2010).
G. Oertel, Polyurethane Handbook, Hanser, New York, 1985.
P. Thomas, Polyurethanes, 2nd ed., Wiley & Sons, Chichester, 1998, Vol. III.
Z. Ranjbar, S. Montazeri, M. M. R. Nayini, and A. Jannesari, Prog. Org. Coat., 69, 426 (2010).
R. W. Seymour and S. L. Cooper, Macromolecules, 6, 48 (1973).
M. G. Smolka, L. Haubler, and D. Fischer, Thermochim. Acta, 351, 95 (2000).
G. Woods, The ICI Polyurethane Book, 2nd ed., Wiley & Sons, Chichester, 1990.
A. S. Nasar, S. Subramani, and G. Radhakrishnan, Polym. Int., 48, 614 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, T., Lu, M. & Liang, L. Synthesis and properties of biodegradable polyurethane crosslinkers from methyl ethyl ketoxime-blocked diisocyanate. Macromol. Res. 20, 827–834 (2012). https://doi.org/10.1007/s13233-012-0113-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-012-0113-3