Abstract
A mycological survey of fungi, present in several stages of the manufacturing of cork discs for champagne stoppers in Portugal, was made. Sixty-nine strains belonging to the Glabra series of the genus Penicillium were isolated and subsequently grouped according to their partial β-tubulin gene sequences. Six groups with different partial β-tubulin gene sequences were observed, and a selection of isolates of each group was made. These selected isolates and various related ex-type strains were subjected to a taxonomical study using a polyphasic approach. This approach included analysis of macro- and microscopic features, the comparison of extrolite profiles and sequencing a part of the β-tubulin and calmodulin gene. The six β-tubulin types were reduced to three different species. One group of isolates was centred on the ex-type strain of P. glabrum, a second group accommodated the type strain of P. spinulosum and a third group contained isolates which were unique in their β-tubulin and calmodulin sequences, extrolite profiles and growth characteristics. This group of isolates is described as the new species Penicillium subericola. The type strain of P. subericola CBS 125096T was isolated from Portuguese raw cork, but additional isolates were found from soil, air and lumen.
Similar content being viewed by others
Introduction
Cork is the outer bark of the cork oak tree (Quercus suber). It is the most suitable material for cork stoppers, due to its unique properties, such as elasticity, compressibility and impermeability to gas or liquids (Lopes et al. 2001; Mano 2002). During a survey of the colonizing mycobiota of cork slabs along the industrial manufacture of cork stoppers, numerous Penicillium isolates were isolated and identified using morphological characters. More than half of the isolates belonged to the Glabra series, and were present in all production stages. However, identification of the different isolates up to species level appeared to be difficult due the high similarities in macro- and micromorphology.
Raper and Thom (1949) placed P. glabrum (as P. frequentans), P. spinulosum and P. purpurescens in the P. frequentans series, and later this series was synonymised with the Glabra series by Pitt (1979). The Glabra series was created to accommodate the fast growing Penicillia with monoverticillate conidiophores and contains eight species (P. chermesinum, P. sclerotiorum, P. donkii, P. decumbens, P. thomii, P. glabrum, P. spinulosum and P. purpurescens). Among those species, P. glabrum and P. spinulosum were morphologically similar and could be best differentiated based on conidial ornamentation. However, the morphological resemblance has caused much confusion and isolates are often misidentified or not differentiated by taxonomists using morphological and physiological techniques (Pitt et al. 1990).
Sixty-nine strains originating from cork and belonging to the Glabra series were grouped according to their partial β-tubulin gene sequences. A subset of these strains was selected for macro- and microscopic analysis, extrolite profiling and sequencing a part of the β-tubulin and calmodulin gene. In addition, ex-type strains of various related species were included in the analysis. Our polyphasic taxonomic approach shows that a group of isolates share peculiar differences with other known species, and a new species is proposed for this group of isolates.
Materials and methods
Fungal strains
For our taxonomic study, a selection of these sixty-nine strains isolated from cork, was made and supplemented with related (ex-type) strains (Table 1). Spore suspensions of the cultures were maintained in 20% glycerol at −80°C.
Sequencing and data analysis
The strains were grown for 2–3 days at 25°C on malt peptone medium. Genomic DNA was isolated using the Ultraclean™ Microbial DNA Isolation Kit (MoBio, Solana Beach, U.S.A.) according the manufacturer’s instructions. Fragments, containing a part of the β-tubulin or calmodulin gene, were amplified and subsequently sequenced according the procedure previously described (Houbraken et al. 2007). The alignments and analyses were preformed as described by Samson et al. (2009). Newly obtained sequences were deposited in Genbank nucleotide sequence database under GQ367499-369547, GU372883-GU372894 and GU991606-GU991609.
Phenotypic identification
All strains were grown on malt extract agar (MEA, Oxoid), Czapek Yeast autolysate agar (CYA), creatine agar (CREA) and Yeast Extract Sucrose agar (YES) (Samson et al. 2010). These media were inoculated in a three-point position and incubated at 25°C for 7 days. In addition, CYA plates were incubated at 30°C and 37°C. After incubation, the culture characteristics were recorded. Microscopic characters were determined on MEA and CYA.
Extrolite extraction and analysis
A selection of ten cork isolates was made based on the results of the β-tubulin analysis, and subjected to extrolite profiling. In addition, various related ex-type strains were examined. The extrolite extractions from the culture media were preformed according to the methods described by Frisvad and Thrane (1987) and Smedsgaard (1997), using 500 μL ethylacetate/methanol/dichloromethane 3:2:1 (vol./vol./vol.) with 1% formic acid. The mixture was ultrasonicated in a bath for 60 min. The organic solvent was transferred to a new vial and evaporated in a fume hood for 24 h. The extract was re-dissolved in 400 μL methanol, analysed by HPLC with diode array detection (DAD) and the extrolites were identified by their UV spectra and retention times.
Results
Grouping of members of the Glabra series isolated from cork
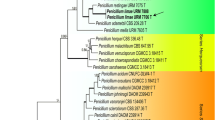
The genetic variation within the strains isolated from cork was investigated using the partial β-tubulin sequences. The strains isolated from cork and four ex-type strains (P. glabrum, P. frequentans, P. paczoskii and P. spinulosum) were added to the dataset, and subjected to an UPGMA analysis (Sneath and Sokal 1973). The sum of branch length of the optimal tree was 0.1301 and the dendrogram is shown in Fig. 1. In total, 422 positions were present in the final dataset. Six groups could be identified among the cork isolates belonging to the Glabra series. The largest group (50 isolates) shared the same partial β-tubulin sequence with the type of P. glabrum, CBS 125543 (Group 1). One cork isolate (CBS 127703) appeared to have a unique partial β-tubulin sequence differing from other isolates in this clade (group 2). Group 4 was the second largest group and consisted of 14 isolates. This group was closely related with group 3 (3 isolates) and these two groups only differed by one base pair. Group 5 and 6 were deviating from the other groups and the β-tubulin data shows that members of group 6 share sequences with the type of P. spinulosum. Group 5 contained one isolate and this strain will be described here as a new species P. subericola. Each unique sequence type was compared by a BLAST search in the NCBI database with the P. glabrum strains identified by Serra et al. (2008). In total three P. glabrum sequences were deposited by Serra et al. (2008) and NRRL 35621 appeared to have identical sequences as “group 2”, while the other two sequences (NRRL 35626 and NRRL 35684) were unique and not assignable to any of our groups. A selection of strains was made and the isolates presented in bold in Fig. 1 were used for a detailed polyphasic study.
Phylogenetic analysis
A combined dataset with partial β-tubulin and calmodulin gene sequences was analysed using RAxML (Fig. 2). The alignment had 230 distinct patterns and the proportion of gaps and completely undetermined characters in the alignment was 0.0302. The phylogenetic analysis showed that there were two main well supported clades. In one clade P. spinulosum, P. palmense and P. subericola were present and in the other clade P. glabrum, and P. purpurescens were located. Penicillium purpurescens was basal to P. glabrum and the P. glabrum isolates were divided in two groups. In one group the majority of the cork isolates were located, together with the type strain of P. glabrum and the ex-type strains of P. flavidorsum, P. spinuloramigenum, P. terlikowskii, P. trzebinskii and P. oledzskii. The other group consisted of the type strains of P. frequentans and P. paczowskii. In the other clade, P. palmense was basal to P. spinulosum and P. subericola. The ex type of P. palmense clustered together with P. grancanariae CBS 687.77T.
Penicillium spinulosum and P. subericola were on a branch with a fair bootstrap support (72%). Three groups were detected within this clade, but none of the phylogenetic relations between those groups were well supported. The isolates of P. subericola were on one branch. Interestingly, P. spinulosum was divided in two groups. One group compasses the type culture of this species and the type strains of P. mucosum CBS 269.35 and P. tannophilum CBS 271.35; the other group contained the type strains of P. mediocre CBS 268.35 and P. tannophagum CBS 289.36.
Phenotypic analysis
The strains isolated from cork were inoculated on the agar media MEA, CYA 25°C, CYA30°C, CYA 37°C, CREA and YES and were compared with the type strains of P. glabrum, P. spinulosum, P. frequentans and P. paczoskii. None of the examined strains were able to grow on CYA incubated at 37°C. In Fig. 3 an overview is shown of growth patterns on various agar media. There was a large variation in macromorphology among the Glabra strains. The type strain of P. glabrum and P. spinulosum were deviating and showed reduced growth rates and weak sporulation. The reverse colours on CYA of the Glabra members were in shades of orange or orange brown, and occasionally in crème colours. The intensity of these colours varied per isolate and ranged from pale orange-brown to vivid orange or red-orange (in P. spinulosum). The variation observed among the Glabra cork isolates could not clearly be correlated to any of the six groups previously assigned with the partial β-tubulin data. No clear distinctive characters to differentiate between P. glabrum, P. spinulosum and the new species could be observed on CYA, MEA and YES. However, there was a striking difference on creatine agar. Isolates of P. spinulosum and the new species P. subericola grew moderate to good on this medium and the majority of both species produced base compounds after prolonged incubation. The colony diameter was generally larger than 25 mm, while P. glabrum isolates grew more restricted (often less than 25 mm) Fig. 3.
Microscopic analysis of the strains showed that P. glabrum, P. spinulosum and P. subericola sp. nov. were very similar to each other. All species were predominantly monoverticillate, with vesiculate conidiophores and 6–12 ampulliform phialides. The main microscopical difference was the conidia ornamentation, which was smooth to slightly rugose in P. glabrum and P. subericola sp. nov., and distinctly rugose in P. spinulosum. Moreover, the conidia of P. subericola tended to be more rugose than in P. glabrum and the conidiophores of this species occasionally were branched, a character not observed in P. glabrum and P. spinulosum.
Extrolites analysis
The majority of the strains assigned to P. glabrum, P. spinulosum and P. subericola produced a pattern of extrolites typical for each species (see Table 2). The P. glabrum isolates had a typical extrolites profile containing asterric acid, bisdechlorogeodin, sulochrin or citromycetin, while isolates of P. spinulosum produce asperfuran, palitantin and frequentin. Asperfuran, deoxybrevianamide E and unidentified compounds which were tentatively named AMF were found in the P. subericola. These AMF compounds are indols with an extended chromophore similar to penitremone. Two cork isolates which phylogenetically clearly belong to P. glabrum (CBS 126333 and 127701) were chemically weak and show no detectable extrolite production.
Discussion
The majority of cork isolates were identified as P. glabrum using the current taxonomical schemes. Four different sequence types of β-tubulin within P. glabrum could be detected. BLAST searches on the NCBI database and local databases of the CBS-Fungal Biodiversity Centre showed that many more sequence types are present in P. glabrum. This intra-species β-tubulin variation is in contrast with species in subgenus Penicillium, where various species share the same tubulin sequence (Samson et al. 2004). The large variability among P. glabrum isolates originating from cork is also observed using microsatellite primers (Basílio et al. 2006). Our analysis show that P. flavidorsum, P. spinuloramigenum, P. terlikowskii, P. trzebinskii and P. oledzskii are synonyms of P. glabrum.
Raper and Thom (1949) placed P. glabrum (P. frequentans), P. spinulosum and P. purpurescens in the P. frequentans series. Our data show that these three species are phylogenetic related. Pitt (1979) named this the Glabra series and expanded it with Penicillia, which have monoverticillate penicilli and a colony diameter on CYA larger than 30 mm after 7 days at 25°C. Penicillium chermesinum, P. sclerotiorum, P. donkii, P. decumbens, P. thomii, P. glabrum, P. spinulosum and P. purpurescens were included, but the phylogenetic analysis of the genus Penicillium by Peterson (2000) showed that the former four species were not closely related to P. glabrum. Furthermore, Peterson (2000) named this monophyletic clade “Group 2”, and showed that the species E. pinetorum, P. asperosporum, P. lividum and E. lapidosum were related to P. glabrum. These findings in a large extent supported in our study, but there are some differences. The taxonomic position of E. lapidosum warrants further attention. This species was not included in our phylogenetic study because the type strain of this species (CBS 343.48) is phylogenetically unrelated to the Glabra group (J. Houbraken, unpublished data). This is in contrast with the observation made by Peterson (2000), which stated that E. lapidosum was conspecific with P. thomii.
Our data show that P. palmense and P. grancanariae, both isolated from air in Gran Canaria, Spain (Ramirez et al. 1978), are synonymous. The type strains of P. frequentans and P. paczowskii were considered to be synonyms of P. glabrum and P. spinulosum respectively (Pitt, 1979). However, based on calmodulin, tubulin and RPB2 data (data not shown) both type strains are placed in a separate clade related to P. glabrum, suggesting that P. frequentans/P. paczowskii and P. glabrum are two distinct species. This evidence is also supported by the extrolites profiles of these species (Frisvad, unpublished data).
Phenotypical differences were observed between the type strains and the cultures isolated from the cork. This is probably due to the fact that the type strains are maintained in cultures collections for a considerable period. Gradual degeneration of various traits due to long-term maintenance and sub culturing are reported. Also degeneration could be due to the lyophilization process, and colony characteristics could be affected due to a lower survival of spores in lyophilised cultures, compared to the fresh cultures (Okuda et al. 1990). The main distinction between P. glabrum and P. spinulosum was the conidia wall texture, which was smooth to finely rugose in P. glabrum and finely roughened to distinctly spinose in P. spinulosum. Some isolates belonging to the Glabra series were difficult to identify correctly even by skilled taxonomists (Pitt et al. 1990). However, to overcome this problem molecular and chemical techniques combined with classical taxonomy were analysed together here, giving a more accurate answer to the taxonomic position of these closely related species. In this study we show that P. glabrum can be differentiated from P. spinulosum and P. subericola by its weak growth on creatine agar.
The concept of exo-metabolome was introduced by Thrane et al. (2007) to enclose all the metabolites produced by fungi in interaction with the environment. The cork isolates belonging to the Glabra series could be grouped in three different extrolite profiles. One similar to the type strain of P. glabrum, a second group produced extrolites in common with the type strain of P. spinulosum and a third one characteristic of P. subericola. Two isolates were chemically weak and did not produce any extrolites. This might be due to degeneration by long-term maintenance, sub-culturing or lack of selection pressure from the environment. The non-production of expected metabolites could also be due to some (point) mutations on the regulatory gene (Larsen et al. 2005). Moreover, P. spinulosum cork isolates produced also some metabolites that were not characteristic of the species, although some of them were described in some P. spinulosum isolates. Since the production of secondary metabolites is more or less genus or species specific (Frisvad et al. 1998, 2008) the existence of P. glabrum cork isolates that produced two different extrolite profiles indicated the existence of intraspecific variability.
The species concept, based not only on DNA sequences, but also in ecological, phenotypic characters and exo-metabolome profiles provide a more accurate and real classification, as verified by studies on Penicillium subgenus Penicillium (Samson and Frisvad 2004) and black Aspergilli (Samson et al. 2007). Applying this polyphasic approach, P. spinulosum and P. subericola can be regarded as two separate species. Hoff et al. (2008) suggested in their study of P. chrysogenum that closely related species could be mating types of the same biological species. However, no differences in extrolite patterns and phenotype could be observed in isolates of different mating types of Paecilomyces variotii (Houbraken et al. 2008, Samson et al. 2009). Furthermore, our studies showed that the two mating types discovered in Aspergillus fumigatus (O’Gorman et al. 2009) and Penicillium chrysogenum (Hoff et al. 2008) produced the same pattern of extrolites and are identical in their phenotype (Houbraken, Samson and Frisvad, unpublished data). In case of P. subericola we have observed differences in both growth patterns and extrolite production and hence the description of a new species is warranted.
The cork isolates now classified as P. glabrum species showed a high intraspecific variability. The macro- and micromorphologies, extrolites profiles and results of the sequencing of partial regions of the β-tubulin and calmodulin genes supported that variability. If the results were analyzed separately (e.g. the extrolite profile and β-tubulin sequencing) probably some of them could indicate the existence of at least two different species. The analysis of more isolates of this species isolated from different sources and from different geographic locations is needed to determine species boundaries in P. glabrum and related species.
Penicillium subericola Baretto, Frisvad & Samson, sp. nov.—Mycobank MB 517383 - Fig. 4.
Penicillio glabro simile, sed bene crescenti in agaro creatino et formatione mixtionis chemicae obscurae (sed in P. glabro non producenti) distinguitur.
Culture ex type: CBS 125096, ex raw cork, Portugal
Colony diameters at 7 days in mm: CYA at 25 º C: 37–44; CYA at 30°C: 16–34; CYA at 37°C: no growth; MEA 35–42; YES 39–46; CREA 14–26, moderate to good growth with moderate to good acid production, base production after prolonged incubation (14 days).
Good sporulation on CYA, grey-green, velvety and floccose in centre, non sporulating margins 1–6 mm, few small hyaline exudates droplets present, reverse colour cream to brownish. Colonies on MEA grey-green, good sporulation, floccose some isolates with velvety colonies and/or velvety with floccose in the centre, exudate absent, reverse is orange brown. Colonies on YES in various shades of green-grey, none or weak sporulation, mycelium inconspicuous, white margins with 1–2 mm, exudates absent, reverse orange-brown to yellow-brown, strongly sulcated (wrinkled).
Conidiophores strictly monoverticillate, stipes vesiculate up to 6 μm, smooth, occasionally short 40 μm, majority longer, width 3.0–4.0, vesicles 4.5–7.0 μm, phialides flask shaped, 10–14 × 2.0–3.0 μm, conidia globose, finely roughened, 3–3.5 μm.
Extrolites: asperfuran, deoxybrevianamide E and unidentified compounds which are indols with an extended chromophore similar to penitremone.
Other isolates examined: CBS 127706 ex-lumber, Vancouver, BC, Canada; CBS 125100 = IBT 30068, from dried grapes (sultanas, Vitis vinifera), Mildura, Vic, Australia; CBS 125099 = IBT 20218 and CBS 125098 = IBT 20217, both from acidified lake, Butte, Montana.
References
Basílio MC, Gaspar R, Silva Pereira C, San Romão MV (2006) Penicillium glabrum cork colonising isolates—preliminary analysis of their genomic similarity. Rev Iberoam Micol 23:151–154
Frisvad JC, Thrane U (1987) Standardized high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). J Chromatogr 404:195–214
Frisvad JC, Thrane U, Filtenborg O (1998) Role and use of secondary metabolites in fungal taxonomy. In: Frisvad JC, Bridge PD, Arora DK (eds) Chemical fungal taxonomy. Marcel Dekker, New York, pp 289–319
Frisvad JC, Andersen B, Thrane U (2008) The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res 112:231–240
Hoff B, Pöggeler S, Kück U (2008) Eighty years after its discovery, Fleming’s Penicillium strain discloses the secret of its sex. Eukaryotic Cell 7:465–470
Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA (2007) Polyphasic taxonomy of Aspergillus section Usti. Stud Mycol 59:107–128
Houbraken J, Varga J, Rico-Munoz E, Johnson S, Samson RA (2008) Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph: Paecilomyces variotii). Appl Environ Microbiol 74:1613–1619
Larsen T, Smedsgaard J, Nielsen K, Hansen M, Frisvad J (2005) Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat Prod Rep 22:672–695
Lopes M, Barros A, Neto C, Rutledge D, Delgadillo I, Gil A (2001) Variability of cork from Portuguese Quercus suber studied by solid-state C-13-NMR and FTIR spectroscopies. Biopolymers 62:268–277
Mano J (2002) The viscoelastic properties of cork. J Mat Sci 37:257–263
O'Gorman CM, Fuller HT, Dyer PS (2009) Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474
Okuda T, Klich MA, Seifert K, Ando K (1990) Media and incubation effects on morphological characteristics of Penicillium and Aspergillus. In: Samson R, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Plenum Press, New York, pp 83–99
Peterson SW (2000) Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Samson R, Pitt J (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood, Reading, pp 163–178
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic, London
Pitt JI, Klich MA, Shaffer GP, Cruickshank RH, Frisvad JC, Mullaney EJ, Onions AHS, Samson RA, Williams AP (1990) Differentiation of Penicillium glabrum from Penicillium spinulosum and other closely related species: an integrated taxonomic approach. System Appl Microbiol 13:304–309
Ramirez C, Martinez AT, Ferrer S (1978) Three new species of Penicillium. Mycopathol 66:77–82
Raper KB, Thom C (1949) Manual of the Penicillia. Williams and Wilkins, Baltimore
Samson RA, Frisvad JC (2004) Penicillium subgenus Penicillium: new taxonomic schemes, mycotoxins and other extrolites. Stud Mycol 49:1–174
Samson RA, Seifert KA, Kuijpers AFA, Houbraken JAMP, Frisvad JC (2004) Phylogenetic analysis of Penicillium subgenus Penicillium using partial b-tubulin sequences. Stud Mycol 49:175–200
Samson RA, Noonim P, Meijer M, Houbraken J, Frisvad JC, Varga J (2007) Diagnostic tools to identify black Aspergilli. Stud Mycol 59:129–145
Samson RA, Houbraken J, Varga J, Frisvad JC (2009) Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia 22:14–27
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2010) Food and Indoor Fungi. CBS Laboratory Manual Series 2. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands
Serra R, Peterson S, CTCOR VA (2008) Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Res Microbiol 159:178–86
Smedsgaard J (1997) Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatogr A 760:264–270
Sneath PHA, Sokal RR (1973) Numerical Taxonomy. Freeman, San Francisco
Thrane U, Andersen B, Frisvad J, Smedsgaard J (2007) The exo-metabolome in filamentous fungi in Topics in Current Genetics. Vol 18. In: Nielsen J, Jewett MC (eds), Metabolomics. pp 235–252
Acknowledgments
This research received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP6 "Structuring the European Research Area" Programme. Carmo Barreto thanks Fundação para a Ciência e Tecnologia for the grant BD/19264/2004. Keith Seifert and John Pitt kindly provided strains and Tineke van Doorn and Martin Meijer are greatly acknowledged for their excellent technical support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Barreto, M.C., Houbraken, J., Samson, R.A. et al. Taxonomic studies of the Penicillium glabrum complex and the description of a new species P. subericola . Fungal Diversity 49, 23–33 (2011). https://doi.org/10.1007/s13225-011-0090-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-011-0090-4