Abstract
Introduction
Noninvasive prenatal testing (NIPT) has revolutionized prenatal screening for chromosomal aneuploidies in some countries. Its implementation has been sporadic in developing countries. Given the genetic variation of the people in different countries, we evaluated the performance of the SNP-based NIPT in India .
Materials and Methods
The Panorama™ NIPT was performed in 516 pregnancies, which had tested intermediate-to-high risk on conventional first and second trimester screening. Results were confirmed either by invasive diagnostic testing or by clinical evaluation after birth.
Results
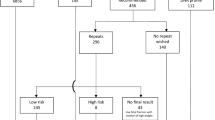
Of 511 samples analyzed, results were obtained in 499 (97.7%). Of these, 480 (98.2%) were low risk and 19 were high risk. A sensitivity of 100% was obtained for detection of trisomies 21, 18, 13 and sex chromosomal abnormalities. The specificity ranged from 99.3 to 100% for abnormalities tested. Taken together, the positive predictive value for trisomies 21, 18, 13 and monosomy X was 85.7%. The average fetal fraction was 8.2%, which is lower than the average observed elsewhere.
Conclusion
This is the first report of detailed experience with NIPT in India and demonstrates comparable performance in all aspects of testing to the results elsewhere.




Similar content being viewed by others
References
Verma IC. Noninvasive prenatal testing: the Indian perspective. J Fetal Med. 2014;1:113–8.
Guanciali-Franchi P, Iezzi I, Palka C, et al. Comparison of combined, stepwise sequential, contingent, and integrated screening in 7292 high-risk pregnant women. Prenat Diagn. 2011;31(11):1077–81.
Russo ML, Blakemore KJ. A historical and practical review of first trimester aneuploidy screening. Semin Fetal Neonatal Med. 2014;19(3):183–7.
Padula F, Cignini P, Giannarelli D, et al. Retrospective study evaluating the performance of a first-trimester combined screening for trisomy 21 in an Italian unselected population. J Prenat Med. 2014;8(3–4):50–6.
Norton ME, Rink BD. Changing indications for invasive testing in an era of improved screening. Semin Perinatol. 2016;40(1):56–66. https://doi.org/10.1053/j.semperi.2015.11.008 (Epub 2015 Dec 24. Review).
Akolekar R, Beta J, Picciarelli G, et al. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16–26.
Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
Norton ME, Brar H, Weiss J, et al. Non-invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–8.
Bianchi DW, Platt LD, Goldberg JD, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901.
Nicolaides KH, Syngelaki A, Ashoor G, et al. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. YMOB. 2012;207(374):e1–6.
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–97.
Larion S, Warsof SL, Romary L, et al. Association of combined first-trimester screen and noninvasive prenatal testing on diagnostic procedures. Obstet Gynecol. 2014;123(6):1303–10.
Dondorp W, De Wert G, Bombard Y, et al. Non-invasive prenatal testing for aneuploidy and beyond: challenges of responsible innovation in prenatal screening. Eur J Hum Genet. 2015;23(11):1438–50.
Zimmermann B, Hill M, Gemelos G, et al. Non-invasive prenatal aneuploidy testing at chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat Diagn. 2012;32(13):1233–41.
Samango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non- invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–9.
Curnow KJ, Wilkins-Haug L, Ryan A, et al. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism-based noninvasive prenatal test. Am J Obstet Gynecol. 2015;212(1):79e1–9.
Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212(3):332.e1–9.
Gross SJ, Stosic M, McDonald-McGinn DM, et al. Clinical experience with a SNP-based noninvasive prenatal test for 22q11.2 deletion syndrome. Ultrasound Obstet Gynecol. 2016;47:177–83.
Pergament E, Cuckle H, Zimmermann B, et al. Single-nucleotidepolymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet Gynecol. 2014;124(21):210–8.
Dar P, Curnow KJ, Gross SJ, et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol. 2014;211(5):527.e1–17.
Dar P, Shani H, Evans MI. Cell-f ree DNA comparison of technologies. 2016;1–13.
Ryan A, Hunkapiller N, Banjevic M, et al. Validation of an enhanced version of a single -nucleotide polymorphism-based noninvasive prenatal test for detection of fetal aneuploidies. Fetal Diagn Ther. 2016;40(3):219–23.
Gregg AR, Gross SJ, Best RG, et al. Noninvasive Prenatal Screening Work Group of the American College of Medical Genetics and Genomics: ACMG statement on noninvasive pre-natal screening for fetal aneuploidy. Genet Med. 2013;15(5):395–8.
Benn P, Borrell A, Crossley J, et al. Aneuploidy screening: a position statement from a committee on behalf of the Board of the International Society for Prenatal Diagnosis, January 2011. Prenat Diagn. 2011;31(6):519–22.
Benn P, Borell A, Chiu R, et al. Position statement from the Aneuploidy Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2013;33(7):622–9.
Benn P, Borrell A, Chiu RW, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2015;35(8):725–34.
Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive pre-natal screening for fetal aneuploidy, update: a position statement of the American College. Genet Med. 2016;2016:1–10.
Tamang R, Thangaraj K. Genomic view on the peopling of India. Investig Genet. 2012;3(20):1–9.
Hamamy H. Consanguineous marriages preconception consultation in primary health care settings. J Community Genet. 2012;3(3):185–92.
Gupta V. Genomic efficiency of endogamy in India. Int J Hum Genet. 2011;11(3):199–201.
Ministry of Health and Family Welfare, Government of India: The Pre-natal Diagnostic Techniques (Regulation and Prevention of Misuse Act. 1994;57 and The Pre-natal Diagnostic Techniques (Regulation and Prevention of Misuse) Amendment Act, 2002 (No. 14 of 2003).
Ashoor G, Syngelaki A, Wagener M, et al. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–5.
Stokowski R, Wang E, White K, et al. Clinical performance of non-invasive prenatal testing (NIPT) using targeted cell-free DNA analysis in maternal plasma with microarrays or next generation sequencing (NGS) is consistent across multiple controlled clinical studies. Prenat Diagn. 2015;35(12):1243–6.
Porreco RP, Garite TJ, Maurel K, et al. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol. 2014;211(4):365.e1–12.
Lutgendorf MA, Stoll KA, Knutzen DM, et al. Noninvasive prenatal testing: limitations and unanswered questions. Genet Med. 2014;16(4):281–5.
Revello R, Sarno L, Ispas A, et al. Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result. Ultrasound Obstet Gynecol. 2016;47(6):698–704.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Rasaily R, Mathur A, Singh MP. Feasibility of introducing genetic services in the National Family Welfare Programme in India. Indian J Med Res. 2009;130(4):404–12.
Verma IC, Dua-Puri R, Bijarnia-Mahay S. ACMG 2016 update on noninvasive prenatal testing for fetal aneuploidy: implications for India. J Fetal Med. 2017;4:1–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Dr. Ramprasad VL, Dr. Priya Kadam, Dr. Venkataswamy E, Shruti Lingaiah, Riyaz Akhtar, Francis Kidangan, Chandran R., Kiran C., and Ravi Kumar G. R. were/are employed with Medgenome Laboratories Private Limited during the course of the project.
Ethical approval
The study was reviewed and approved by ethical review board of the institutions that participated in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Prof. I.C. Verma is the HOD in the Department of Centre of Medical Genetics Sir Ganga Ram Hospital, New Delhi, India; Ratna Puri is Professor and Chairperson, Institute of Medical Genetic and Genomics, Sir Ganga Ram Hospital, New Delhi, India; Eswarachary Venkataswamy is Associate Director Q&A, Medgenome Labs Pvt. Ltd, Bengaluru, India; Tulika Tayal is Maternal and Fetal Medical Consultant, Rainbow Hospital, Hyderabad, India; Sheela Nampoorthiri is Clinical Professor, Department of Paediatric Genetics, Amrita Institute of Medical Sciences and Research Center, Kochi, India; Chitra Andrew is Senior Consultant, Obstetrics and Gynecology, Sri Ramachandra Medical College, Chennai, India; Madhulika Kabra is Additional Professor and Officer-in-Charge, Genetic Unit, All India Institute of Medical Sciences, New Delhi, India; Rashmi Bagga is Professor, Obstetrics and Gynecology, Postgraduate Institute and Medical Research Center, Chandigarh, India; Mamatha Gowda is Assistant Professor, Obstetrics and Gynecology, Jawaharlal Nehru Institute of Postgraduate Medical Education and Research, Pondicherry, India; Meenu Batra is Consultant Radiologist, Feto-maternal Medicine, CIMAR Fertility Center, Kochi, India; Sridevi Hegde is Head, Department of Medical Genetics, Manipal Hospital, Bengaluru, India; Anita Kaul is Senior Consultant and Coordinator, Indraprastha Apollo Hospital, New Delhi, India; Neerja Gupta is Assistant Professor, Division of Genetics, All India Institute of Medical Sciences, New Delhi, India; Pallavi Mishra is Biochemist, All India Institute of Medical Sciences, New Delhi, India; Jayshree Ganapathi Subramanian is NIPT Project Coordinator, Sir Ganga Ram Hospital, New Delhi, India; Shruti Lingaiah is Senior Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Riyaz Akhtar is Senior Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Francis Kidangan is Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Chandran R. is Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Kiran C is Senior Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Ravi Kumar GR is Research Associate, Medgenome Labs Pvt. Ltd, Bengaluru, India; Ramprasad V.L. is the Chief Operating Officer, Medgenome Labs Pvt. Ltd, Bengaluru, India; Priya Kadam is NIPT Project Director, Medgenome Labs Pvt. Ltd, Bengaluru, India.
Rights and permissions
About this article
Cite this article
Verma, I.C., Puri, R., Venkataswamy, E. et al. Single Nucleotide Polymorphism-Based Noninvasive Prenatal Testing: Experience in India. J Obstet Gynecol India 68, 462–470 (2018). https://doi.org/10.1007/s13224-017-1061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-017-1061-9