Abstract
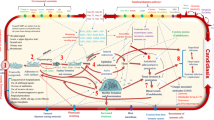
Pseudomonas aeruginosa is an increasingly prevalent opportunistic pathogen that causes a variety of nosocomial infections, life-threatening diseases in immunocompromised persons and chronic pulmonary infections in cystic fibrosis patients. The organism’s virulence depends on an arsenal of cell-associated and extracellular factors determining the pathogenesis of infections as multifactorial. Most P. aeruginosa infections are both invasive and toxinogenic. Many of the extracellular virulence factors (proteases, exotoxin A, pyocyanin, siderophores, hemolysins) required for tissue invasion and dissemination of P. aeruginosa are controlled by quorum sensing (QS) that enable the bacteria to produce these factors in a coordinated, cell-density-dependent manner and overwhelm the host defense mechanisms during acute infection. Sometimes, QS also contributes to biofilm formation and thus participates in pathogenesis of chronic infection. This system is recognized to be a global regulatory network controlling the expression of a large number of virulence genes either directly or indirectly. Two-component sensor kinases such as RetS, LadS and GacS are also controlling the production of virulence factors as well as the switch from acute to chronic infection. The present review describes the known virulence determinants of P. aeruginosa, the stages of infection as well as the importance of QS in the pathogenesis of P. aeruginosa infection.


Similar content being viewed by others
References
Alcorn JF, Wright JR (2004) Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J Biol Chem 279:30871–30879
Alhede M, Bjarnsholt T, Jensen PØ, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Høiby N, Rasmussen TB, Givskov M (2009) Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155:3500–3508
Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB (2000) Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect Immun 68:3998–4004
Arhin A, Boucher C (2010) The outher membrane protein OprQ and adherence of Pseudomonas aeruginosa to human fibronectin. Microbiology 156:1415–1423
Arts J, van Boxtel R, Filloux A, Tommassen J, Koster M (2007) Export of the pseudopilin XcpT of the Pseudomonas aeruginosa type II secretion system via the signal recognition particle-Sec pathway. J Bacteriol 189:2069–2076
Baitch AL, Obrig TG, Snith RP, Hammer MC, Conroy JV, Lutz F (1987) Production of cytotoxin by clinical strains of Pseudomonas aeruginosa. Can J Microbiol 33:104–111
Ball G, Durand E, Lazdunski A, Filloux A (2002) A novel type II secretion system in Pseudomonas aeruginosa. Mol Microbiol 43:475–485
Baltch AL, Hammer MC, Smith RP, Obrig TG, Conroy JV, Bishop MB, Egg MA, Lutz F (1985) Effects of Pseudomonas aeruginosa cytotoxin on human serum and granulocytes and their microbicidal, phagocytic, and chemotactic functions. Infect Immun 48:498–506
Barbieri AM, Sha Q, Bette-Bobillo P, Stahl PD, Vidal M (2001) ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect Immun 69:5329–5334
Berka RM, Vasil ML (1982) Phospholipase C (heat labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol 152:239–245
Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, Tolker-Nielsen T, Givskov M, Hoiby N, Ciofu O, Scandinavian Cystic Fibrosis Study Consortium (2010) Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 5(4):e10115. doi:10.1371/journal.pone.0010115
Blanc DS, Petignat C, Janin B, Bille J, Francioli P (1998) Frequency and molecular diversity of Pseudomonas aeruginosa upon admission and during hospitalization: a prospective epidemiologic study. Clin Microbiol Infect 4:242–247
Bleves S, Viarre V, Salacha R, Michel GPF, Filloux A, Voulhoux R (2010) Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int J Med Microbiol 300:534–543
Britigan BE, Railsback MA, Cox CD (1999) The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: implications for the pathogenesis of the cystic fibrosis lung disease. Infect Immun 67:1207–1212
Bryan R, Kube D, Perez A, Davis P, Prince A (1998) Overproduction of the CFTR R domain leads to increased levels of asialoGM1 and increased Pseudomonas aeruginosa binding by epithelial cells. Am J Respir Cell Mol Biol 19:269–277
Cacalano G, Kays M, Saiman L, Prince A (1992) Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Invest 89:1866–1874
Cantón R, Cobos N, de Gracia J, Baquero F, Honorato J, Gartner S, Alvarez A, Salcedo A, Oliver A, García-Quetglas E, Spanish Consensus Group for Antimicrobial Therapy in the Cystic Fibrosis Patient (2005) Antimicrobial therapy for pulmonary pathogenic colonization and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect 11:690–703
Castric PA, Deal CD (1994) Differentiation of Pseudomonas aeruginosa pili based on sequence and B-cell epitope analyses. Infect Immun 62:371–376
Chemani C, Imberty A, de Bentzmann S, Pierre M, Wimmerová M, Guery BP, Faure K (2009) Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun 77:2065–2075
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Cowell BA, Chen DY, Frank DW, Vallis AJ, Fleiszig SMJ (2000) ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun 68:403–406
Cox CD (1980) Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol 142:581–587
Cystic Fibrosis Foundation (2009) Patient registry: 2008 Annual Data Report. Cystic Fibrosis Foundation, Bathesda
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costeron JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of bacterial biofilm. Science 280:295–298
Davies J, Dewar A, Bush A, Pitt T, Gruenert D, Geddes DM, Alton EW (1999) Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1 antibody and neuraminidase inhibition. Eur Respir 13:565–570
De Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski MC, Puchelle E (1996) AsialoGM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun 64:1582–1588
Denning GM, Railsback MA, Rasmussen GT, Cox CD, Britigan BE (1998) Pseudomonas pyocyanine alters calcium signaling in human airway epithelial cells. Am J Physiol 274:L893–L900
Deziel E, Lepine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG (2004) Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci USA 101:1339–1344
Diggle SP, Cornelis P, Williams P, Cámara M (2006a) 4-quinolone signaling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol 296:83–91
Diggle SP, Stacey RE, Dodd C, Cámara M, Williams P, Winzer K (2006b) The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol 8:1095–1104
Dobrindt U, Hochhut B, Hentschel U, Hacker J (2004) Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol 2:414–424
Duong F, Bonnet E, Geli V, Lazdunski A, Murgier M, Filloux A (2001) The AprX protein of Pseudomonas aeruginosa: a new substrate for the Apr type I secretion system. Gene 262:147–153
Durand E, Bernadac A, Ball G, Lazdunski A, Sturgis JN, Filloux A (2003) Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrilar and adhesive structure. J Bacteriol 185:2749–2758
Durand E, Verger D, Rêgo AT, Chandran V, Meng G, Fronzes R, Waksman G (2009) Structural biology of bacterial secretion systems in Gram-negative pathogens – potential for new drug targets. Infect Disord Drug Targets 9:518–547
Engel J, Balachandran P (2009) Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol 12:61–66
Farinha MA, Conway BD, Glasier LM, Ellert NW, Irvin RT, Sherburne R, Paranchych W (1994) Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect Immun 62:4118–4123
Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A (1998) Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66:43–51
Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR (2001) Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659–2669
Filloux A (2009) The type VI secretion system: a tubular story. EMBO J 28:309–310
Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW (1997) ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 25:547–557
Finck-Barbancon V, Yahr TL, Frank DW (1998) Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J Bacteriol 180:6224–6231
Finnan S, Morrissey JP, O’Gara F, Boyd EF (2004) Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J Clin Microbiol 42:5783–5792
Fuqua C, Winans SC, Greenberg EP (1996) Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators (review). Annu Rev Microbiol 50:727–751
Galloway DR (1991) Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol 5:2315–2321
Gambello MJ, Iglewski BH (1991) Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol 173:3000–3009
Gambello MJ, Kaye S, Iglewski BH (1993) LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun 61:1180–1184
Ganesan AK, Frank DW, Misra RP, Schmidt G, Barbieri JT (1998) Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem 273:7332–7337
Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel JN (2000) The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun 68:7100–7113
Garrity-Ryan L, Shafikhani S, Balachandran P, Nguyen L, Oza J, Jakobsen T, Sargent J, Fang X, Cordwel S, Matthay MA, Engel JN (2004) The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect Immun 72:546–558
Geiser T, Kazmierczak B, Garrity-Ryan L, Matthay M, Engel J (2001) Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell Microbiol 3:223–236
Gilboa-Garber N (1982) Pseudomonas aeruginosa lectins. Methods Enzymol 83:378–385
Glick J, Garber N (1983) The intracellular localization of Pseudomonas aeruginosa lectins. J Gen Microbiol 129:3085–3090
Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT (1999) The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem 274:36369–36372
Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S (2009) Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev 23:249–259
Guzzo J, Pages JM, Duong F, Lazdunski A, Murgier M (1991) Pseudomonas aeruginosa alkaline protease: evidence for secretion genes and study of secretion mechanism. J Bacteriol 173:5290–5297
Hachani A, Lossi NS, Hamilton A et al. (2011) Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J Biol Chem. Epub ahead of print as Manuscript M110.193045
Hahn HP (1997) The type-4 pilus is the major virulence-associated adhesion of Pseudomonas aeruginosa – a review. Gene 192:99–108
Hauser A (2009) The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7:654–665
Hauser AR, Engel JN (1999) Pseudomonas aeruginosa induces type III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun 67:5530–5537
Hauser AR, Kang PJ, Engel JN (1998) Pep A, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol 27:807–818
Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J (2002) Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30:521–528
He J, Baldini RL, Deziel E, Saucier M, Zhang Q, Liberati NT, Lee D, Urbach J, Goodman HM, Rahme LG (2004) The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc Natl Acad Sci USA 101:2530–2535
Heck LW, Morihara K, McRae WB, Miller EJ (1986) Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun 51:115–118
Heck LW, Alarcon PG, Kulhavy RM, Morihara K, Russel MW, Mestecky JF (1990) Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol 6:2253–2257
Hogardt M, Roeder M, Schreff AM, Eberl L, Heesemann J (2004) Expression of Pseudomonas aeruginosa exoS is controlled by quorum sensing and RpoS. Microbiology 150:843–851
Hong Y, Ghebrehiwet B (1992) Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin Immunol Immunopathol 62:133–138
Howe TR, Iglewski BH (1984) Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun 43:1058–1063
Jacquot J, Tournier J, Puchelle E (1985) In vitro evidence that human airway lysozyme is cleaved and inactivated by Pseudomonas aeruginosa elastase and not by human leukocyte elastase. Infect Immun 47:555–560
Jaffar-Bandjee MC, Lazdunski A, Bally M, Carrere J, Chazalette JP, Galabert C (1995) Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol 33:924–929
Johnson DA, Carter-Hamm B, Dralle WM (1982) Inactivation of human bronchial mucous proteinase inhibitor by Pseudomonas aeruginosa elastase. Am Rev Respir Dis 126:1070–1073
Juhas M, Eberl L, Tümmler B (2005) Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol 7:459–471
Kamath JM, Britigan BE, Cox CD, Shasby DM (1995) Pyocyanin from Pseudomonas aeruginosa inhibits prostacyclin release from endothelial cells. Infect Immun 63:4921–4923
Kazmierczak BI, Engel JN (2002) Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42.Infect Immun 70:2198–2205
Kernacki KA, Hobden JA, Hazlett LD, Fridman R, Berk RS (1995) In vivo bacterial protease production during Pseudomonas aeruginosa corneal infection. Investig Ophthalmol Vis Sci 36:1371–1378
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508
Kida Y, Higashimoto Y, Inoue H, Shimizu T, Kuwano K (2008) A novel secreted protease from Pseudomonas aeruginosa activates NF-kappaB through protease-activated receptors. Cell Microbiol 10:1491–1504
Kleerebezem M, Quadri LE, Kuipers OP, de Vos VM (1997) Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria (review). Mol Microbiol 24:895–904
Kluftinger JL, Lutz F, Hancock REW (1987) Pseudomonas aeruginosa cytotoxin: periplasmic localization and inhibition of macrophages. Infect Immun 57:882–886
Krall R, Schmidt G, Aktories K, Barbieri JT (2000) Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect Immun 68:6066–6068
Krall R, Sun J, Pederson K, Barbieri J (2002) In vivo Rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect Immun 70:360–367
Kubota Y, Liu PV (1971) An enterotoxin of Pseudomonas aeruginosa. J Infect Dis 123:97–98
Kulasekara BR, Kulasekara DH, Wolfgang MC, Stevens L, Frank DW, Lory S (2006) Acquisition and evolution of the exoU locus in Pseudomonas aeruginosa. J Bacteriol 188:4037–4050
Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP (1999) Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 104:743–750
Lanotte P, Watt S, Mereghetti L, Dartiguelongue N, Rastegar-Lari A, Goudeau A, Quentin R (2004) Genetic feature of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J Med Microbiol 53:73–81
Laskowski MA, Osborn E, Kazmierczak BI (2004) A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol Microbiol 54:1090–1103
Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A (1996) A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol 21:1137–1146
Laughlin RS, Musch MW, Hollbrook CJ, Rocha FM, Chang EB, Alverdy JC (2000) The key role of Pseudomonas aeruginosa PA-I lectin on experimental gut-derived sepsis. Ann Surg 232:133–142
Lazdunski AM, Ventre I, Sturgis JM (2004) Regulatory circuits and communication in Gram-negative bacteria. Nat Rev Microbiol 2:581–592
Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK (2005) The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol 175:7512–7518
Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ (2009) Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci USA 106:4154–4159
Leprat R, Michel-Briand Y (1980) Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann Microbiol 131B:209–222
Letoffe S, Redeker V, Wandersman C (1998) Isolation and characterization of an extracellular haem-binding protein from Pseudomonas aeruginosa that shares function and sequence similarities with the Serratia marcescens HasA haemophore. Mol Microbiol 28:1223–1234
Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, Kim KC (2001) Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 280:L181–L187
Lillehoj EP, Kim BT, Kim KC (2002) Identification of Pseudomonas aeruginosa flagellin as an adhesion for Muc1 mucin. Am Physiol Lung Cell Mol Physiol 282:L751–L756
Liu PV (1974) Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis 130:S94–S99
Liu PV (1979) Toxins of Pseudomonas aeruginosa. In: Doggett RG (ed) Pseudomonas aeruginosa. Clinical manifestations of infection and current therapy. Academic, New York, pp 63–88
Lu HM, Motley ST, Lory S (1997) Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol Microbiol 25:247–259
Lutz F, Maurer M, Failing K (1987) Cytotoxic protein from Pseudomonas aeruginosa: formation of hydrophilic pores in Ehrlich ascites tumor cells and effect on cell viability. Toxicon 25:293–304
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA (2003) A genetic basis for Pseudomonas aeruginosa biofilm antimicrobial resistance. Nature 426:306–310
Manz-Keinke H, Plattner H, Schlepper-Schafer J (1992) Lung surfactant protein A (SP-A) enhances serum-independent phagocytosis of bacteria by alveolar macrophages. Eur J Cell Biol 57:95–100
Mariencheck WI, Savov J, Dong Q, Tino MJ, Wright JR (1999) Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of Pseudomonas aeruginosa. Am J Physiol 277:L777–L786
Mariencheck WI, Alcorn JF, Palmer SM, Wright JR (2003) Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol 28:528–537
Mattick JS (2002) Type IV pili and twitching motility. Annu Rev Microbiol 56:289–314
Merrell DS, Falkow S (2004) Frontal and stealth attack strategies in microbial pathogenesis. Nature 430:250–256
Michel GP, Voulhoux R (2009) The type II secretory system (T2SS) in Gram-negative bacteria: a molecular nanomachine for secretion of Sec and Tat-dependent extracellular proteins. In: Wooldridge KG (ed) Bacterial secreted proteins: secretory mechanisms and role in pathogenesis. Caister, Norfolk, pp 67–92
Middlebrook JL, Dorland RB (1977) Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol 23:183–189
Misfeldt ML, Legard PK, Howell SE, Fornella MH, LeGrand RD (1990) Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun 58:978–982
Morihara K, Tsuzuki H, Oda K (1979) Protease and elastase of Pseudomonas aeruginosa: inactivation of human α-1-proteinase inhibitor. Infect Immun 24:188–193
Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordoñez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ (2006) A virulence locus of P. aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530
Mühlradt PF, Tsai H, Conradt P (1986) Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur J Immunol 16:434–440
Nicas TI, Bradley J, Lochner JE, Iglewski BH (1985a) The role of exoenzyme S in infections with Pseudomonas aeruginosa. J Infect Dis 152:716–721
Nicas TI, Frank DW, Stenzel P, Lile JD, Iglewski BH (1985b) Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur J Clin Microbiol 4:175–179
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
Ochsner UA, Reiser J (1995) Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92:6424–6428
Ochsner UA, Koch AK, Fiechter A, Reiser J (1994) Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176:2044–2054
Olson JC, Fraylick JE, McGuffie EM, Dolan KM, Yahr TL, Frank DW, Vincent TS (1999) Interruption of multiple cellular processes in HT-29 epithelial cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun 67:2847–2854
Ostroff RM, Vasil AI, Vasil ML (1990) Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J Bacteriol 172:5915–5923
Parmely M, Gale A, Clabaugh M, Horvat R, Zhou WW (1990) Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun 58:3009–3014
Pearson JP, Gray KM, Passador L, Tucker KD, Ebehard A, Iglewski BH, Greenberg EP (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91:197–201
Pearson JP, Passador L, Iglewski BH, Greenberg EP (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92:1490–1494
Pederson KJ, Barbieri JT (1998) Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas exoenzyme S is cytotoxic to eukaryotic cells. Mol Microbiol 30:751–760
Pederson KJ, Vallis AJ, Aktories K, Frank DW, Barbieri JT (1999) The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol 32:393–401
Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132
Pesci EC, Milbank JBJ, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH (1999) Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 96:11229–11234
Phillips RM, Six DA, Dennis EA, Ghosh P (2003) In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem 278:41326–41332
Pinheiro MRS, Lacerda HR, Melo RGL, Maciel MA (2008) Pseudomonas aeruginosa infections: factors relating to mortality with emphasis on resistance pattern and antimicrobial treatment. Braz J Infect Dis 12:509–515
Pollack M (1995) Pseudomonas aeruginosa. In: Mandell GL, Benett JE, Dolin R (eds) Principles and practice of infectious diseases, 4th edn. Churchill Livingstone, New York, pp 1980–2003
Qiu X, Gurkar AU, Lory S (2006) Interstrain transfer of the large pathogenicity island (PAPI-1) of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 103:19830–19835
Rabin SDP, Hauser AR (2005) Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect Immun 73:573–582
Rabin SDP, Veesenmeyer JL, Bieging KT, Hauser AR (2006) A C-terminal domain targets the Pseudomonas aeruginosa cytotoxin ExoU to the plasma membrane of host cells. Infect Immun 74:2552–2561
Ramphal R, Small PM, Shands JW, Fischlschweiger W Jr, Small PA Jr (1980) Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun 27:614–619
Ramphal R, Arora SK, Ritchings BW (1996) Recognition of mucin by the adhesin-flagellar system of Pseudomonas aeruginosa. Am J Respir Crit Care Med 154:S170–S174
Ramsey BW (1996) Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med 335:179–188
Ran H, Hassett DJ, Lau GW (2003) Human targets of Pseudomonas aeruginosa pyocyanin. Proc Natl Acad Sci USA 100:14315–14320
Read RC, Roberts P, Munro N (1992) Effects of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol 72:2271–2277
Restrepo CI, Dong Q, Savov J, Mariencheck WI, Wright JR (1999) Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am J Respir Cell Mol Biol 21:576–585
Ruer S, Stender S, Filloux A, de Bentzmann S (2007) Assembly of fimbrial structures in Pseudomonas aeruginosa: functionality and specificity of chaperone-usher machineries. J Bacteriol 189:3547–3555
Ruer S, Ball G, Filloux A, de Bentzmann S (2008) The ‘P-usher’, a novel protein transporter involved in fimbrial assembly and TpsA secretion. EMBO J 27:2669–2680
Rumbaugh KP, Griswold JA, Hamood AN (2000) The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect 14:1721–1731
Saiman L, Prince A (1993) Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest 92:1875–1880
Sasak T, Lutz F (1985) Action of a cytotoxin from Pseudomonas aeruginosa on human leukemic cell lines. FEBS Lett 189:33–36
Schelstraete P, Van Daele S, De Boeck K, Proesmans M, Lebecque P, Leclercq-Foucart J, Malfroot A, Vaneechoutte M, De Baets F (2008) Pseudomonas aeruginosa in the home environment of newly infected cystic fibrosis patients. Eur Respir J 31:822–829
Schmidt H, Hensel M (2004) Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev 17:14–56
Schulert GS, Feltman H, Rabin SDP, Battle SE, Rello J, Hauser AR (2003) Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis 188:1695–1706
Schuster M, Lostroh CP, Ogi T, Greenberg EP (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079
Schuster M, Hawkins AC, Harwood CS, Greenberg EP (2004) The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol 51:973–985
Seed PC, Passador L, Iglewski BH (1995) Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol 177:654–659
Semmler AB, Whitchurch CB, Mattick JS (1999) A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology 145:2863–2873
Sherbrock-Cox V, Russell NJ, Gacesa P (1984) The purification and chemical characterisation of the alginate present in the extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res 135:147–154
Shilo M (1957) The breakdown of the lactose derivate by bacteria. Biochem J 66:48–49
Shriniwas MU, Bhujwala RA (1979) Production of permeability factor and enterotoxin by Pseudomonas aeruginosa. Indian J Med Res 70:380–383
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP (2000) Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762–764
Smith RS, Iglewski BH (2003) Pseudomonas aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 6:56–60
Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV (2006) Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103:8487–8492
Sonawane A, Jyot J, Ramphal R (2006) Pseudomonas aeruginosa LecB is involved in pilus biogenesis and protease IV activity but not in adhesion to respiratory mucins. Infect Immun 74:7035–7039
Soong G, Muir A, Gomez MI, Waks J, Reddy B, Planet P, Singh PK, Kanetko Y, Wolfgang MC, Hsiao Y-S, Tong L, Prince A (2006) Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest 116:2297–2305
Spangenberg C, Fislage R, Sierralta W, Tummler B, Romling U (1995) Comparison of type IV-pilin genes of Pseudomonas aeruginosa of various habitats has uncovered a novel unusual sequence. FEMS Microbiol Lett 125:265–273
Stickler DJ, Morris NS, McLean RJC, Fuqua C (1998) Biofilms on indwelling urethral catheters produce quorum sensing signal molecules in situ and in vitro. Appl Environ Microbiol 64:3486–3490
Stover CK, Pham XQ, Erwin AL et al (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Strateva T, Petrova G, Perenovska P, Mitov I (2009) Bulgarian cystic fibrosis Pseudomonas aeruginosa isolates: antimicrobial susceptibility and neuraminidase-encoding gene distribution. J Med Microbiol 58:690–692
Strateva T, Markova B, Ivanova D, Mitov I (2010) Distribution of the type III effector proteins-encoding genes among nosocomial Pseudomonas aeruginosa isolates from Bulgaria. Ann Microbiol 60:503–509
Strom MS, Lory S (1993) Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol 47:565–596
Strom MS, Nunn DN, Lory S (1993) A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA 90:2404–2408
Suttorp N, Seeger W, Uhl J, Lutz F, Roka L (1985) Pseudomonas aeruginosa cytotoxin stimulates prostacyclin production in cultured pulmonary artery endothelial cells: membrane attack and calcium influx. J Cell Physiol 123:64–72
Tielker D, Hacker S, Loris R, Strathmann M, Wingender J, Wilhelm S, Rosenau F, Jaeger KE (2005) Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology 151:1313–1323
Todar K (2009) Opportunistic infections caused by Pseudomonas aeruginosa. In: Kenneth Todar’s Online Textbook of Bacteriology. University of Wisconsin-Madison, Department of Bacteriology, http://www.textbookofbacteriology.net/
Toder DS, Gambello MJ, Iglewski BH (1991) Pseudomonas aeruginosa LasA; a second elastase gene under transcriptional control of lasR. Mol Microbiol 5:2003–2010
Twining SS, Kirschner SE, Mahnke LA, Frank DW (1993) Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig Ophthalmol Vis Sci 34:2699–2712
Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK (2002) Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol 168:1861–1868
Vallis AJ, Finck-Barbancon V, Yahr TL, Frank DW (1999) Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun 67:2040–2044
Van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4:551–560
Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A (2006) Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci USA 103:171–176
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30:274–291
Vidal DR, Garrone P, Banchereau J (1993) Immunosuppressive effects of Pseudomonas aeruginosa exotoxin A on human B-lymphocytes. Toxicon 31:27–34
Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM (2004) Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev 68:132–153
Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095
Wandersman C, Delepelaire P (2004) Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58:611–647
Wentworth JS, Austin FE, Garber NC, Gilboa-Garber N, Paterson CA, Doyle RJ (1991) Cytoplasmic lectins contribute to the adhesion of Pseudomonas aeruginosa. Biofouling 4:99–104
Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25:365–404
Whiteley M, Lee KM, Greenberg EP (1999) Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 96:13904–13909
Whiteley M, Parsec MR, Greenberg EP (2000) Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J Bacteriol 182:4356–4360
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864
Wick MJ, Hamood AN, Iglewski BH (1990) Analysis of the structure-function relationship of Pseudomonas aeruginosa exotoxin A (review). Mol Microbiol 4:527–535
Wilhelm S, Tommassen J, Jaeger KE (1999) A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol 181:6977–6986
Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE (2007) The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J Bacteriol 189:6695–6703
Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ (1988) Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun 56:2515–2517
Winzer K, Falconer C, Garber NC, Diggle SP, Camara M, Williams P (2000) The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J Bacteriol 182:6401–6411
Woods DE, Iglewski BH (1983) Toxins of Pseudomonas aeruginosa: new perspectives. Rev Infect Dis 5:714–722
Yahr TL, Barbieri JT, Frank DW (1996a) Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol 178:1412–1419
Yahr TL, Goranson J, Frank DW (1996b) Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol 22:991–1003
Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW (1998) ExoY, a novel adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95:13899–13904
Zehavi-Wilner T (1988) Induction of murine cytotoxic T-lymphocytes by Pseudomonas aeruginosa exotoxin A. Infect Immun 56:213–218
Zhang L, Mah TF (2008) Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 190:4447–4452
Zhang L, Wang H, Masters SC, Wang B, Barbieri JT, Fu H (1999) Residues of 14–3–3 zeta required for activation of exoenzyme S of Pseudomonas aeruginosa. Biochemistry 38:12159–12164
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strateva, T., Mitov, I. Contribution of an arsenal of virulence factors to pathogenesis of Pseudomonas aeruginosa infections. Ann Microbiol 61, 717–732 (2011). https://doi.org/10.1007/s13213-011-0273-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0273-y