Abstract
The ability to predict the transglycosylation activity of glycosidases by in silico analysis was investigated. The transglycosylation abilities of 7 different β-d-galactosidases from GH family 2 were tested experimentally using 7 different acceptors and p-nitrophenyl-β-d-galactopyranoside as a donor of galactosyl moiety. Similar transglycosylation abilities were confirmed for all enzymes originating from bacteria belonging to Enterobacteriaceae, which were able to use all tested acceptor molecules. Higher acceptor selectivity was observed for all others used bacterial strains. Structure models of all enzymes were constructed using homology modeling. Ligand-docking method was used for enzymes-transglycosylation products models construction and evaluation. Results obtained by in silico analysis were compared with results arisen out of experimental testing. The experiments confirmed that significant differences in transglycosylation abilities are caused by small differences in active sites composition of analyzed enzymes. According to obtained result, it is possible to conclude that homology modeling may serve as a quick starting point for detection or exclusion of enzymes with defined transglycosylation abilities, which can be used for subsequent synthesis of e.g., pharmaceutically interesting glycosides.



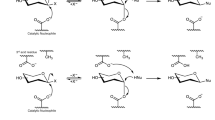
taken from E. coli; b Loop formed by residues 414–427 (red) in the enzyme from L. delbrueckii subsp. bulgaricus (orange, in comparison with E. coli in blue) and c Substitution of His357 (E. coli numbering, histidine residues of all enzymes are in orange) by Leu (blue) in the enzyme from Enterobacter cloacae
Similar content being viewed by others
References
Bartesaghi A, Merk A, Banerjee S, Matthies D, Wu X, Milne JL, Subramaniam S (2015) 2.2 Å resolution cryo-EM structure of β-galactosidase in complex with a cell-permeant inhibitor. Science 348:1147–1151. https://doi.org/10.1126/science.aab1576
Bojarová P, Křen V (2011) Glycosidases in carbohydrate synthesis: when organic chemistry falls short. Chimia 65:65–70. https://doi.org/10.2533/chimia.2011.65
Brás NF, Fernandes PA, Ramos MJ (2010) QM/MM studies on the β-galactosidase catalytic mechanism: hydrolysis and transglycosylation reactions. J Chem Theory Comput 6(2):421–433. https://doi.org/10.1021/ct900530f
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision Glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49:6177–6196. https://doi.org/10.1021/jm051256o
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644. https://doi.org/10.1016/S0959-440X(97)80072-3
Jorgensen WL (2004) The many roles of computation in drug discovery. Science 303:1813–1818. https://doi.org/10.1126/science.1096361
Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW (2020) A structural view of the action of Escherichia coli (Lacz) Beta-galactosidase. Biochemistry 40:14781–14794. https://doi.org/10.1021/bi011727i
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The Carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495
Lu L, Guo L, Wang K, Liu Y, Xiao M (2020) β-Galactosidases: A great tool for synthesizing galactose-containing carbohydrates. Biotechnol Adv 39:107465. https://doi.org/10.1016/j.biotechadv.2019.107465
Materials Science Suite (2018) Schrödinger. LLC, New York, NY, p 2018
Miguez Amil S, Jimenez-Ortega E, Ramirez-Escudero M, Talens-Perales D, Marin-Navarro J, Polaina J, Sanz-Aparicio J, Fernandez-Leiro R (2020) The cryo-EM structure of Thermotoga maritima beta-Galactosidase: quaternary structure guides protein engineering. ACS Chem Biol 15:179–188. https://doi.org/10.1021/acschembio.9b00752
Naumoff DG (2011) Hierarchical classification of glycoside hydrolase. Biochemistry (Moscow) 76:622–635. https://doi.org/10.1134/S0006297911060022
Nelson DL, Cox MM (2005) Lehninger principles of biochemistry, 4th edn. W. H. Freeman & Co., New York, NY
Pagadala NS, Syed K, Tuszynski J (2017) Software for molecular docking: a review. Biophys Rev 9:91–102. https://doi.org/10.1007/s12551-016-0247-1
Pawlak-Szukalska A, Wanarska M, Popinigis AT, Kur J (2014) A novel cold-active β-d-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB—Gene cloning, purification and characterization. Process Biochem. 49(12):2122–2133. https://doi.org/10.1016/j.procbio.2014.09.018
Rutkiewicz M, Bujacz A, Wanarska M, Wierzbicka-Wos A, Cieslinski H (2019a) Active Site Architecture and Reaction Mechanism Determination of Cold Adapted β-d-galactosidase from Arthrobacter sp. 32cB. Int. J. Mol. Sci. 20(17):4301. https://doi.org/10.3390/ijms20174301
Rutkiewicz M, Bujacz A (1867b) Bujacz G (2019) Structural features of cold-adapted dimeric GH2 β-d-galactosidase from Arthrobacter sp. 32cB. Biochim Biophys Acta Proteins Proteomics 9:776–786. https://doi.org/10.1016/j.bbapap.2019.06.001
Rutkiewicz M, Wanarska M, Bujacz A (2020) Mapping the Transglycosylation Relevant Sites of Cold-Adapted β-d-Galactosidase from Arthrobacter sp. 32cB. Int J Mol Sci 21(15):5354. https://doi.org/10.3390/ijms21155354
Saqib S, Akram A, Halim SA, Tassaduq R (2017) Sources of β-galactosidase and its applications in food industry. Biotech 7:1–7. https://doi.org/10.1007/s13205-017-0645-5
Sastry GM, Adzhigirey M, Day T, Annabhimouju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234. https://doi.org/10.1007/s10822-013-9644-8
Šícho M, Svozil D (2017) Molekulové dokování jako nástroj pro virtuální návrh léčiv. Chemické Listy 111:754–759
Sippl MJ (1993) Recognition of errors in three-dimensional structures of proteins. Proteins 17:355–362. https://doi.org/10.1002/prot.340170404
Skálová T, Dohnálek J, Spiwok V, Lipovová P, Vondráčková E, Petroková H, Dušková J, Strnad H, Králová B, Hašek J (2005) Cold-active β-galactosidase from Arthrobacter sp. C2–2 forms compact 660 kDa hexamers: crystal structure at 1.9 Å resolution. J Mol Biol 353:282–294. https://doi.org/10.1016/j.jmb.2005.08.028
Wang LX, Huang W (2009) Enzymatic transglycosylation for glycoconjugate synthesis. Curr Opin Chem Biol 13:592–600. https://doi.org/10.1016/j.cbpa.2009.08.014
Webb B, Sali A (2016) Comparative Protein Structure Modeling Using Modeller. Curr Protocols Bioinf 54:5.6.1-5.6.37. https://doi.org/10.1002/cpbi.3
Author information
Authors and Affiliations
Contributions
EB: draft of the manuscript, interpretation of the acquired data. ZS: performing of in silico analysis, homology modeling and molecular docking. MT: performing of experimental analyses of transglycosylation abilities of studied enzymes. VS: design of in silico experiments, interpretation of the acquired data. PL: design of transglycosylation experiments, interpretation of the acquired data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benešová, E., Šućur, Z., Těšínský, M. et al. Transglycosylation abilities of β-d-galactosidases from GH family 2. 3 Biotech 11, 168 (2021). https://doi.org/10.1007/s13205-021-02715-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02715-w