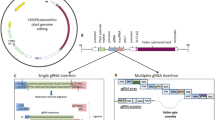
Abstract
A large number of computational tools have been documented in recent years for identification of target-specific valid single-guide (sg) RNAs (18–20 nucleotide long sequence) that is an important component for the efficient utilization of the CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats—CRISPR-associated Protein) system. Despite optimization of Cas9, other major concerns are on-target efficiency and off-target activity that depend upon the sequence(s) of target-specific sgRNA(s). However, a very little attention has been paid for identification of the best-hit sgRNA for precise targeting as well as minimizing the off-target effects. The aim of this present work is to offer comparative insight into existing CRISPR software tools with their unique features (including targeted genome) and utilities. These available web tools were found to be designed based upon only a few limited mathematical models. Among all these available web tools, three (Benchling, Desktop and CRISPR-P) have been curated as exclusively available for plant genome-editing purpose. These three software tools have been comprehensively described and analyzed with single same target enquiry from two randomly selected genes (IDM2 and IDM3 from Arabidopsis thaliana). Interestingly, all these selected tools generated different results (sgRNAs) even for the same query. In fact, the sequence of sgRNA is considered an important parameter to determine the efficiency and specificity of sgRNAs for precise genome editing. Thus, there is an urgent requirement to pay attention for a validated sgRNA-designing tool for precise DNA editing in plants. In conclusion, this work will encourage building up a consensus for developing a universal valid sgRNA designing for different organisms including plants.



Similar content being viewed by others
Abbreviations
- Cas:
-
CRISPR-associated protein/gene
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- sgRNA:
-
Single-guide RNA
- crRNA:
-
CRISPR RNA
- PAM:
-
Protospacer adjacent motif
- CFD:
-
Cutting frequency determination
- ZFN:
-
Zinc finger nuclease
- CLD:
-
CRISPR library designer
- TALEN:
-
Transcription activator-like effector nuclease
- tracRNA:
-
Trans-activating CRISPR RNA
- DESKGEN:
-
Desktop genetics
- NGS:
-
Next-generation sequencing
References
Bae S, Park J, Kim JS (2014) Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475
Bassett AR, Liu JL (2014) CRISPR-Cas9 and genome editing in Drosophila. J Genet Genom 41:7–19
Bhowmik P, Ellison E, Polley B et al (2018) Targeted mutagenesis in wheat microspores using CRISPR-Cas9. Sci Rep 8:6502
Briner AE, Donohoue PD, Gomaa AA et al (2014) Guide RNA functional modules direct Cas9 activity and orthogonality. Mol Cell 56:333–339
Cai Y, Chen L, Shi S et al (2018) CRISRP-Cas9-mediated deletion of large genomic fragments in soyabean. Int J Mol Sci 19:3835
Chari R, Mali P, Moosburner M, Church GM (2015) Unraveling CRISPR-Cas9 genome engineering parameters via a library-on-library approach. Nat Methods 12:823
Chen H, Choi J, Bailey S (2014) Cut site selection by the two nuclease domains of the Cas9 RNA guided endonuclease. J Biol Chem 289:13284–13294
Chen R, Xu Q, Liu Y et al (2018) Generation of transgene-free maize male sterile lines using the CRISPR-Cas9 System. Front Plant Sci 9:1180
Cong L, Ran FA, Cox D et al (2013) Multiplex genome engineering using CRISPR-Cas systems. Science 339:819–823
Doench JG, Hartenian E, Graham DB et al (2014) Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 32:1262–1267
Doench JG, Fus IN, Sullender M et al (2016) Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 34:184–191
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096
Duan CG, Wang X, Xie S et al (2017) A pair of transposon-derived proteins function in a histone acetyltransferase complex for active DNA demethylation. Cell Res 27:226–240
Fagerlund RD, Staals RHJ, Fineran PC (2015) The Cpf1 CRISPR-Cas protein expands genome-editing tools. Genome Biol 16:251
Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, Zhu JK (2013) Efficient genome editing in plants using a CRISPR-Cas system. Cell Res 23:1229
Feng C, Yuan J, Wang R et al (2016) Efficient targeted genome modification in maize using CRISPR-Cas9 system. J Genet Genom 43:37–43
Fine EJ, Cradick TJ, Zhao CL, Lin Y, Bao G (2014) An online bioinformatics tool predicts zinc finger and TALE nuclease off-target cleavage. Nucleic Acids Res 42:e42
Fogarty NM, McCarthy A, Snijders KE et al (2017) Genome editing reveals a role for OCT4 in human embryogenesis. Nature 550:67
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284
Gang L, Zhang H, Lou D, Yu D (2016) Selection of highly efficient sgRNAs for CRISPR-Cas9-based plant genome editing. Sci Rep 6:21451
Gopalappa R, Suresh B, Ramakrishan S, Kim HH (2018) paired D10A Cas9 nickneses are sometimes more efficient than individual nucleases for gene disruption. Nucleic Acids Res 46:e71
Grissa I, Vergnaud G, Pourcel C (2007) CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res 35:W52–W57
Heigwer F, Kerr G, Boutros M (2014) E-CRISP: fast CRISPR target site identification. Nat Methods 11:122–123
Hough SH, Ajetunmobi A, Brody L, Humphryes-Kirilov N, Perello E (2016) Desktop genetics. Per Med 13:517–521
Hsu PD, Scott DA, Weinstein JA, RanFA KonermannS, Agarwala V, Cradick TJ (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827
Jiang F, Doudna JA (2017) CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529
Jinek M, Jiang FG, Taylor DW et al (2014) Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343:1247997
Kim D, Alptekin B, Budak H (2018) CRISPR-Cas9 genome editing in wheat. Funct Integr Genom 18:31–41
Kuan PF, Powers S, He S, Li K, Zhao X, Huang B (2017) A systematic evaluation of nucleotide properties for CRISPR sgRNA design. BMC Bioinform 18:21
Kumar V, Jain M (2014) The CRISPR-Cas system for plant genome editing: advances and opportunities. J Exp Bot 66:47–57
Li Q, Wang X, Sun H, Zeng J, Cao Z, Li Y, Qian W (2015) Regulation of active DNA demethylation by a methyl-CpG-binding domain protein in Arabidopsis thaliana. PLoS Genet 11:e1005210
Li J, Zhang H, Si X et al (2017a) Generation of thermosensitive male-sterile maize by targeted knockout of the ZmTMS5 gene. J Genet Genom 44:465–468
Li WR, Liu CX, Zhang XM, Chen L et al (2017b) CRISPR-Cas9-mediated loss of FGF5 function increases wool staple length in sheep. FEBS 284:2764–2773
Liang G, Zhang H, Lou D, Yu D (2016) Selection of highly efficient sgRNAs for CRISPR-Cas9-based plant genome editing. Sci Rep 6:21451
Liu H, Wei Z, Dominguez A, Li Y, Wang X, Qi LS (2015) CRISPR-ERA: a comprehensive design tool for CRISPR-mediated gene editing, repression, and activation. Bioinformatics 31:3676–3678 (btv423)
Liu X, Homma A, Sayadi J, Yang S, Ohashi J, Takumi T (2016) Sequence feature associated with cleavage efficiency of CRISPR-Cas 9 system. Sci Rep 6:19675
Ma M, Ye AY, Zheng W, Kong L (2013) A guide RNA sequence design platform for the CRISPR-Cas9 system for model organism genomes. Biomed Res Int 2013:1–4
Mendoza BJ, Trinh CT (2017) Enhanced guide-RNA design and targeting analysis for precise CRISPR genome editing of single and consortia of industrially relevant and non-model organisms. Bioinformatics 34:16–23
Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, Wan J, Gu H, Qu L (2013) Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res 23:1233
Miao C, Xiao L, Hua K et al (2018) Mutations in subfamily of abscisic acid receptor genes promote rice growth and productivity. PNAS 115:6058–6063
Mojica FJ, Díez-Villaseñor C, García-Martínez J, Almendros C (2009) Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155:733–740
Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E (2014) CHOPCHOP: a CRISPR-Cas9 and TALEN web tool for genome editing. Nucleic Acids Res 42:W401–W407
Naito Y, Hino K, Bono H, Ui-Tei K (2015) CRISPRdirect: software for designing CRISPR-Cas guide RNA with reduced off-target sites. Bioinformatics 31:1120–1123
Nishimasu H, RanFA Hsu PD, Konermann S, Shehata SI, Dohmae N et al (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156:935–949
Nowak CM, Lawson S, Zerez M, Bleris L (2016) Guide RNA engineering for versatile Cas9 functionality. Nucleic Acids Res 44:9555–9564
Oh JN, Choi KH, Lee CK (2017) Multi-resistance strategy for viral diseases and in vitro shRNA verification method in pigs. Asian Australas J Anim Sci. https://doi.org/10.5713/ajas.17.0749
Park J, Bae S, Kim JS (2015) Cas-Designer: a web-based tool for the choice of CRISPR-Cas9 target sites. Bioinformatics 31:4014–4016
Pellegrini R (2016) Edit single bases with Benchling! https://blog.benchling.com/2016/07/18/base-editor. Accessed 18 July 2016
Periwal V (2016) A comprehensive overview of computational resources to aid in precision genome editing with engineered nucleases. Brief Bioinform 18:698–711
Pliatsika V, Rigoutsos I (2015) Off-Spotter: very fast and exhaustive enumeration of genomic look alikes for designing CRISPR-Cas guide RNAs. Biol Direct 10:4
Plummer RJ, Guo Y, Peng Y (2018) A CRISPR reimagining: new twists and turns of CRISPR beyond the genome-engineering revolution. J Cell Biochem 119:1299–1308
Prykhozhij SV, Rajan V, Gaston D, Berman JN (2015) CRISPR multitargeter: a web tool to find common and unique CRISPR single RNA targets in a set of similar sequences. PLoS One 10:e0138634
Pyott D, Sheehan E, Molnar A (2016) Engineering of CRISPR-Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol 17:1276–1288
Ran FA, Hsu PD, Lin CY et al (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389
Ross MJ, Coates PT (2017) Using CRISPR to inactivate endogenous retroviruses in pigs: an important step toward safe xenotransplantation. Kidney Int 93:4–6
Sánchez-León S, Gil-Humanes J, Ozuna CV et al (2018) Low-gluten, nontransgenic wheat engineered with CRISPR-Cas9. Plant Biotechnol J 16:902–910
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355
Sander JD, Maeder ML, Reyon D, Voytas DF, Joung JK, Dobbs D (2010) ZiFiT (Zinc Finger Targeter): an updated zinc finger engineering tool. Nucleic Acids Res 38:W462–W468
Schwinn MK, Machleidt T, Zimmerman K, Eggers CT, Dixon AS, Hurst R et al (2017) CRISPR-mediated tagging of endogenous proteins with a luminescent peptide. ACS Chem Biol 13:467–474
Shen C, Que Z, Xia Y et al (2017) Rapid generation of genetic diversity by multiplex CRISPR-Cas9 genome editing in rice. Sci China Life Sci 60:89–93
Shi J, Gao H, Wang H et al (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15:207–216
Shimatani Z, Kashojiya S, Takayama M et al (2017) Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat Biotechnol 35:441–443
Shmakov S, Abudayyeh OO, Makarova KS et al (2015) Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell 60:385–397
Slaymaker IM, Gao L, Zetshe B et al (2016) Rationally engineered Cas9 nucleases with improved specificaity. Science 351:84–88
Song J, yang D, Ruan J, Zhang J (2017) Production of immunodeficient rabbits by multiplex embryo transfer and multiplex gene targeting. Sci Rep 7:12202
Sontheimer EJ, Wolfe SA (2015) Cas9 gets a classmate. Nat Biotechnol 33:1240–1241
Stemmer M, Thumgerger T, del Sol Keyer M, Wittbrodt J, Mateo JL (2017) CCTop: an intuitive, flexible and reliable CRISPR-Cas9 target prediction tool. PLoS One 12:e0176619
Tang L, Zeng Y, Du H, Gong M (2017) CRISPR-Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Genet Genom 292:525–533
Ueta R, Abe C, Watanabe T et al (2017) Rapid breeding of parthenocarpic tomato plants using CRISPR-Cas9. Sci Rep 7:507
Uniyal AP, Yadav SK, Kumar V (2019) The CRISPR–Cas9, genome editing approach: a promising tool for drafting defense strategy against begomoviruses including cotton leaf curl viruses. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-019-00491-6
Upadhyay SK, Sharma S (2014) SSFinder: high throughput CRISPR-Cas target sites prediction tool. Biomed Res Int 2014:742482
Vilarino M, Rashid ST, Suchy FP, McNabb BR et al (2017) CRISPR-Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep 12:17472
Wang W, Pan Q, Akhunova A et al (2018) Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J 1:65–74
Wei C, Wang F, Liu W, Zhao W, Yang Y, Li K, Xiao L, Shen J (2018) CRISPR-Cas9 targeting of the androgen receptor suppresses the growth of LNCaP human prostate cancer cells. Mol Med Rep 17:2901–2906
Wright AV, Nunez JK, Doudna JA (2016) Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164:29–44
Wu F, Ge Gao, Pan T, Yang Z et al (2017) Generation of a SMO homozygous knockout human embryonic stem cell line WAe001-A-16 by CRISPR-Cas9 editing. Stem Cell Res 24:89–93
Xiao A, Cheng Z, Kong L, Zhu Z, Lin S, Gao G, Zhang B (2014) CasOT: a genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 30:1180–1182 (btt764)
Xie S, Shen B, Zhang C, Huang X, Zhang Y (2014) sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS One 9:e100448
Yang L, Li L, Hai-Yang L, Sen L, Feng X, Ling-Ling C (2014) CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant 7:1494–1496
Yang Y, Zhu G, Li R (2017) The RNA editing factor SIORRM4 is required for normal fruit ripening in tomato. Plant Physiol 175:1690–1702
Yin K, Gao C, Qiu JL (2017) Progress and prospects in plant genome editing. Nat Plants 3:17107
Zetsche B, Heidenreich M, Mohanraju P et al (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nature Biotechnol 35:178
Zhang Y, Heidrich N, Ampattu BJ et al (2013) Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. Mol Cell 50:488–503
Zhang F, Wen Y, Guo X (2014) CRISPR-Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet 23:R40–R46
Zhang Z, Ge X, Luo X et al (2018a) Simultaneous editing of two copies of Gh14-3-3d confers enhanced transgene-clean plant defense against Verticillium dahliae in allotetraploid upland cotton. Front Plant Sci 9:842
Zhang T, Zheng Q, Yi X et al (2018b) Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol J 16:1415–1423
Zhu LJ, Holmes BR, Aronin N, Brodsky MH (2014) CRISPRseek: a bioconductor package to identify target-specific guide RNAs for CRISPR-Cas9 genome-editing systems. PLoS One 9:e108424
Zou E, Cai YJ, Li K, Wei Y (2017) One-step generation of complete gene knockout mice and monkeys by CRISPR-Cas9-mediated gene editing with multiple sgRNAs. Cell Res 27:933–945
Acknowledgements
The authors express deepest gratitude to Vice Chancellor of Central University of Punjab, India for providing financial support during course of this work. “UGC-BSR start up grant” sanctioned to Vinay Kumar, who sponsors this research. The authors thank anonymous reviewers and editors for critical reading the manuscript and suggesting substantial improvements.
Author information
Authors and Affiliations
Contributions
VK conceived and designed the present research. VK and KM conducted the experiments. KM and VK analyzed the data. APU, SKY and VK wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Uniyal, A.P., Mansotra, K., Yadav, S.K. et al. An overview of designing and selection of sgRNAs for precise genome editing by the CRISPR-Cas9 system in plants. 3 Biotech 9, 223 (2019). https://doi.org/10.1007/s13205-019-1760-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1760-2