Abstract
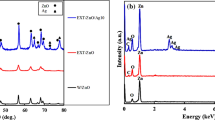
The synthesis and characterization of an aggregate of AgNPs coated with plant extract (PE) from Alphonsea sclerocarpa and its significant antimicrobial activity and inhibition on K562 (blood cancer) cells have been appended in the article. Synthesis of aggregate [(AgNPs)-(PE)] has been followed by a facile eco-friendly approach without using any harmful chemicals. The morphology of an aggregate [(AgNPs)-(PE)] was confirmed by TEM and SEM microscopic characterizations. Properties like solid state, the presence of functional groups, and elemental composition have been characterized through the XRD, FTIR, and EDAX. The biocompatibility of synthesized aggregate of [(AgNPs)-(PE)] was confirmed by the MTT assay. An in vitro cell (HEK293)-based studies were performed for the biocompatibility tests and it is found that the aggregate [(AgNPs)-(PE)] is not harmful to normal/healthy cells. Even though A. sclerocarpa show the antimicrobial (antibacterial and antifungal) activity, it has been further enhanced with the developed aggregate of [(AgNPs)-(PE)]. Furthermore, it has been extended to examine the cellular inhibition on K562 cells and obtained > 75% cell inhibition for 24 h treated cells.







Similar content being viewed by others
References
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28
Amgoth C, Dharmapuri G, Kalle AM, Paik P (2016) Nanoporous capsules of block copolymers of [(MeO-PEG-NH)-b-(L-GluA)]-PCL for the controlled release of anticancer drugs for therapeutic applications. Nanotechnology 27(12):125101
Andrews JM (2001) Determination of minimum inhibitory concentration. J Antimicrob Chemother 48(1):5–16
Dey Biswajit, Saha Rajat, Mukherjee Priyanka (2013) A luminescent-water soluble inorganic co-crystal for a selective pico- molar range arsenic (III) sensor in a water medium. Chem Commun 63:7064–7066
El-Chaghaby GA, Ahmad AF (2011) Biosynthesis of silver nanoparticles using Pistacia lentiscus leaves extract and investigation of their antimicrobial effect. Orient J Chem 27(3):929–936
Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713. https://doi.org/10.1055/s-2006-957563
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on E. coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/c1gc15386b
Iravani S, Zolfaghari B (2013) Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int 2013:639725. https://doi.org/10.1155/2013/639725
Jang SJ, Yang IJ, Tettey CO, Kim KM, Shin HM (2016) In-vitro anticancer activity of green synthesised silver nanoparticles on MCF-7 human breast cancer cells. Mater Sci Eng 68:430–435. https://doi.org/10.1016/j.msec.2016.03.101
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306. https://doi.org/10.3109/07388550903062462
Krishnan R, Arumugam V, Vasaviah SK (2015) The MIC and MBC of silver nanoparticles against Enterococcus faecalis—a facultative anaerobe. J Nanomed Nanotechnol 6(3):285. https://doi.org/10.4172/2157-7439.1000285
Kumar VA, Uchida T, Mizuki T, Nakajima Y, Katsube Y, Hanajiri T, Maekawa T (2016) Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Adv Nat Sci 7(1):015002
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5(4):916–924. https://doi.org/10.1021/pr0504079
Ma CY, Rout MK, Mock WY (2001) Study of oat globulin conformation by Fourier transform infrared spectroscopy. J Agric Food Chem 49:3328–3334
Mallmann EJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PB (2015) Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo 57(2):165–177. https://doi.org/10.1590/S0036-46652015000200011
McFarland J (1907) Nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc 14:1176–1178. https://doi.org/10.1001/jama.1907.25320140022001f
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bacterial effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
Mueller NC, Nowack B (2008) Exposure modelling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Nagababu P, Uma Maheswara Rao V (2017) Pharmacological assessment, green synthesis and characterization of silver nanoparticles of sonneratia apetala Buch.-Ham. leaves. J App Pharm Sci 7(8):175–182
Narayanan KB, Park HH (2014) Antifungal activity of silver nanoparticles synthesised using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur J Plant Pathol 140:185–192. https://doi.org/10.1007/s10658-014-0399-4
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6(11):1–5. https://doi.org/10.4103/0973-7847.95849
Prasad DN (2009) Antioxidant activity of Alphonsea sclerocarpa bark. Res J Pharmacol Pharmacodynamics 1(2):66–69
Protima R, Siim K, Stanislav F, Erwan R (2015) A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mat Sci Eng. https://doi.org/10.1155/2015/682749
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Sastry M, Mayya KS, Bandyopadhyay K (1997) pH-dependent changes in the optical properties of carboxylic acid derivatised silver colloidal particles. Colloids Surf A 127(1–3):221–228. https://doi.org/10.1016/S0927-7757(97)00087-3
Sastry M, Patil V, Sainkar SR (1998) Electrostatically controlled diffusion of carboxylic acid derivatised silver colloidal particles in thermally evaporated fatty amine films. J Phy Chem B 102(8):1404–1410
Shrivastava S, Bera T, Roy A, Singh G, Rama Chandra Rao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:1–9
Siim K, Sander K, Protima R, Mithu G, David W, Erwan R (2016) Biocidal properties study of silver nanoparticles used for application in green housing. Int Nano Lett 6(3):191–197. https://doi.org/10.1007/s40089-016-0186-7
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Song HY, Ko KK, Oh IH, Lee BT (2006) Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater 11(1):58
Suman Joshi DSD, Venkata Rao G, Satya Prasad M, Kishore Babu M, Surya Narayana S, Krishna Satya A (2017) Phytochemical screening and evaluation of antioxidant, antibacterial and antifungal activity of medicinal plant Alphonsea sclerocarpa Thaw. J Pharmacogn Phytochem 6(4):1280–1286
Tacic D, Wannigama GP, Cassels BK, Cave A (1987) Alkaloids of Alphonsea sclerocarpa. J Nat Prod 50(3):518–519. https://doi.org/10.1021/np50051a036
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120. https://doi.org/10.1016/j.jscs.2010.06.004
Acknowledgements
The authors would like to acknowledge DST-FIST, Department of Physics, Acharya Nagarjuna University for providing IR facility, University of Hyderabad, for providing SEM with EDAX and XRD facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both the first and second authors have equal contribution to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doddapaneni, S.J.D.S., Amgoth, C., Kalle, A.M. et al. Antimicrobial and anticancer activity of AgNPs coated with Alphonsea sclerocarpa extract. 3 Biotech 8, 156 (2018). https://doi.org/10.1007/s13205-018-1155-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1155-9