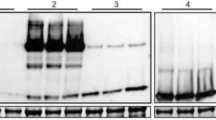
Abstract
The UDP-glucosyltransferase UGT76G1 from Stevia rebaudiana converts stevioside to rebaudioside A via a one-step glycosylation reaction, which increases the amount of sweet-tasting rebaudioside A and decreases the amount of stevioside that has a bitter aftertaste. This enzyme could, therefore, conceivably be used to improve the organoleptic properties of steviol glycosides and offer a cost-effective preparation of high-purity rebaudioside A. Producing soluble enzymes by overexpression is a prerequisite for large-scale biocatalysis. However, most of the UGT76G1 overexpressed in Escherichia coli is in inclusion bodies. In this study, three N-terminal fusion partners, 3′-phosphoadenosine-5′-phosphatase (CysQ), 2-keto-3-deoxy-6-phosphogluconate aldolase (EDA) and N-utilisation substance A (NusA), were tested to improve UGT76G1 expression and solubility in E. coli. Compared with the fusion-free protein, the solubility of UGT76G1 was increased 40% by fusion with CysQ, and the glucosyltransferase activity of the crude extract was increased 82%. This successful CysQ fusion strategy could be applied to enhance the expression and solubility of other plant-derived glucosyltransferases and presumably other unrelated proteins in the popular, convenient and cost-effective E. coli host.






Similar content being viewed by others
References
Adari BR, Alavala S, George SA, Meshram HM, Tiwari AK, Sarma AVS (2016) Synthesis of rebaudioside-A by enzymatic transglycosylation of stevioside present in the leaves of Stevia rebaudiana Bertoni. Food Chem 200:154–158. doi:10.1016/j.foodchem.2016.01.033
Ahn KY, Song JA, Hah KY, Park JS, Seo HS, Lee J (2007) Heterologous protein expression using a novel stress-responsive protein of E. coli RpoA as fusion expression partner. Enzyme Microb Technol 41:859–866. doi:10.1016/j.enzmictec.2007.07.009
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1006/abio.1976.9999
Brandle JE, Telmer PG (2007) Steviol glycoside biosynthesis. Phytochemistry 68:1855–1863. doi:10.1016/j.phytochem.2007.02.010
Chen TH, Zhang Y, Shi ZQ, Liu XH, He BL (1999) Studies on the adsorptive selectivity of the polar resin with carbonyl group on rebaudioside A. Acta Polym Sin 50:398–403
De Marco V, Stier G, Blandin S, de Marco A (2004) The solubility and stability of recombinant proteins are increased by their fusion to NusA. Biochem Biophys Res Commun 322:766–771. doi:10.1016/j.bbrc.2004.07.189
Desmet T, Soetaert W, Bojarova P, Kren V, Dijkhuizen L, Eastwick-Field V, Schiller A (2012) Enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Chemistry 18:10786–10801. doi:10.1002/chem.201103069
Dewitte G, Walmagh M, Diricks M, Lepak A, Gutmann A, Nidetzky B, Desmet T (2016) Screening of recombinant glycosyltransferases reveals the broad acceptor specificity of stevia UGT-76G1. J Biotechnol 233:49–55. doi:10.1016/j.jbiotec.2016.06.034
Georgiou G, Valax P (1996) Expression of correctly folded proteins in Escherichia coli. Curr Opin Biotechnol 7:190–197. doi:10.1016/S0958-1669(96)80012-7
Georgiou G, Valax P (1999) Isolating inclusion bodies from bacteria. Methods Enzymol 309:48–58. doi:10.1016/S0076-6879(99)09005-9
Han KY, Park JS, Seo HS, Ahn KY, Lee J (2008) Multiple stressor-induced proteome responses of Escherichia coli BL21(DE3). J Proteome Res 7:1891–1903. doi:10.1021/pr700631c
Hellman J, Paavilainen S, Mantsala P (1992) Expression in Escherichia coli and purification of intracellular proteins by fusion to cyclomaltodextrin glucanotransferase. J Biotechnol 26:275–288. doi:10.1016/0168-1656(92)90012-X
Jhamb K, Sahoo DK (2012) Production of soluble recombinant proteins in Escherichia coli: effects of process conditions and chaperone co-expression on cell growth and production of xylanase. Bioresour Technol 123:135–143. doi:10.1016/j.biortech.2012.07.011
Jhamb K, Jawed A, Sahoo DK (2008) Immobilized chaperones: a productive alternative to refolding of bacterial inclusion body proteins. Process Biochem 43:587–597. doi:10.1016/j.procbio.2008.02.004
Kane JF (1995) Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol 6:494–500. doi:10.1016/0958-1669(95)80082-4
Kang YS, Song JA, Han KY, Lee J (2015) Escherichia coli EDA is a novel fusion expression partner to improve solubility of aggregation-prone heterologous proteins. J Biotechnol 194:39–47. doi:10.1016/j.jbiotec.2014.11.025
Kim S, Lee SB (2008) Soluble expression of archaeal proteins in Escherichia coli by using fusion-partners. Protein Expr Purif 62:116–119. doi:10.1016/j.pep.2008.06.015
Kolb N, Herrera JL, Ferreyra DJ, Uliana RF (2001) Analysis of sweet diterpene glycosides from Stevia rebaudiana: improved HPLC method. J Agric Food Chem 49:4538–4541. doi:10.1021/jf010475p
Lee JH, Lee JY, Song JA, Han KY, Lee DS, Lee J (2014) A stress-responsive Escherichia coli protein, CysQ is a highly effective solubility enhancer for aggregation-prone heterologous proteins. Protein Expr Purif 101:91–98. doi:10.1016/j.pep.2014.06.006
Li J, Chen ZB, Di DL (2012) Preparative separation and purification of rebaudioside A from Stevia rebaudiana Bertoni crude extracts by mixed bed of macroporous adsorption resins. Food Chem 132:268–276. doi:10.1016/j.foodchem.2011.10.077
Li H, Liu N, Wang WT, Wang JY, Gao WY (2016) Cloning and characterization of GST fusion tag stabilized large subunit of Escherichia coli acetohydroxyacid synthase I. J Biosci Bioeng 121:21–26. doi:10.1016/j.jpiosc.2015.05.010
Lui YF, Di DL, Bai QQ, Li JT, Chen ZB, Lou S, Ye HL (2011) Preparative separation and purification of rebaudioside A from steviol glycosides using mixed-mode macroporous adsorption resins. J Agric Food Chem 59:9629–9636. doi:10.1021/jf2020232
Madhav H, Bhasker S, Chinnamma M (2013) Functional and structural variation of uridine diphosphate glycosyltransferase (UGT) gene of Stevia rebaudiana-UGTSr involved in the synthesis of rebaudioside A. Plant Physiol Biochem 63:245–253. doi:10.1016/j.plaphy.2012.11.029
Neuwald AF, Krishnan BR, Brikun I, Kulakauskas S, Suziedelis K, Tomcsanyi T, Leyh TS, Berg DE (1992) CysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J Bacteriol 174:415–425. doi:10.1128/jb.174.2.415-425.1992
Niiranen L, Espelid S, Karlsen CR, Mustonen M, Paulsen SM, Heikinheimo P, Willassen NP (2007) Comparative expression study to increase the solubility of cold adapted Vibrio proteins in Escherichia coli. Protein Expr Purif 52:210–218. doi:10.1016/j.pep.2006.09.005
Paquette S, Moller BL, Bak S (2003) On the origin of family 1 plant glycosyltransferases. Phytochemistry 62:399–413. doi:10.1016/S0031-9422(02)00558-7
Pasek M, Boeggeman E, Ramakrishnan B, Qasba PK (2010) Galectin-1 as a fusion partner for the production of soluble and folded human beta-1,4-galactosyltransferase-T7 in E. coli. Biochem Biophys Res Commun 394:679–684. doi:10.1016/j.bbrc.2010.03.051
Pei XL et al (2015) Chaperones-assisted soluble expression and maturation of recombinant Co-type nitrile hydratase in Escherichia coli to avoid the need for a low induction temperature. J Biotechnol 203:9–16. doi:10.1016/j.jbiotec.2015.03.004
Raran-Kurussi S, Keefe K, Waugh DS (2015) Positional effects of fusion partners on the yield and solubility of MBP fusion proteins. Protein Expr Purif 110:159–164. doi:10.1016/j.pep.2015.03.004
Rumelhard M, Hosako H, Eurlings IMJ, Westerink WMA, Staska LM, van de Wiel JAG, La Marta J (2016) Safety evaluation of rebaudioside A produced by fermentation. Food Chem Toxicol 89:73–84. doi:10.1016/j.fct.2016.01.005
Schlieker C, Bukau B, Mogk A (2002) Prevention and reversion of protein aggregation by molecular chaperones in the E. coli cytosol: implications for their applicability in biotechnology. J Biotechnol 96:13–21. doi:10.1016/S0168-1656(02)00033-0
Tada A et al (2013) Absolute quantitation of stevioside and rebaudioside A in commercial standards by quantitative NMR. Chem Pharm Bull 61:33–38. doi:10.1248/cpb.c12-00736
Tong YJ, Feng SS, Xin Y, Yang HL, Zhang L, Wang W, Chen W (2016) Enhancement of soluble expression of codon-optimized Thermomicrobium roseum sarcosine oxidase in Escherichia coli via chaperone co-expression. J Biotechnol 218:75–84. doi:10.1016/j.jbiotec.2015.11.018
Urban JD, Carakostas MC, Taylor SL (2015) Steviol glycoside safety: are highly purified steviol glycoside sweeteners food allergens? Food Chem Toxicol 75:71–78. doi:10.1016/j.fct.2014.11.011
Wang Y, Chen L, Li Y, Yan M, Chen K, Hao N, Xu L (2016) Efficient enzymatic production of rebaudioside A from stevioside. Biosci Biotechnol Biochem 80:67–73. doi:10.1080/09168451.2015.1072457
Wolwer-Rieck U (2012) The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: a review. J Agric Food Chem 60:886–895. doi:10.1021/jf2044907
Xu J, Kaloyanova D, Ivanov IG, AbouHaidar MG (1998) The low expression level of pokeweed antiviral protein (PAP) gene in Escherichia coli by the inducible lac promoter is due to inefficient transcription and translation and not to the toxicity of the PAP. Arch Biochem Biophys 351:82–88. doi:10.1006/abbi.1997.0552
Zerbs S, Giuliani S, Collart F (2014) Small-scale expression of proteins in E. coli. Method Enzymol 536:117–131. doi:10.1016/B978-0-12-420070-8.00011-8
Acknowledgements
We greatly acknowledge financial support from the NSFC (21106068), the Open fund Program of the Yichang Key Laboratory of Biocatalysis (2015NP01), Subei Science and Technology Projects (BN2015115), TAPP and Provincial Key R&D Plan of Jiangsu (BE2017703).
Author information
Authors and Affiliations
Contributions
LC and YL conceived and designed the study. LC and PS performed the experiments and analysed the data. LC wrote the paper. MY, LX, KC and PO reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chen, L., Sun, P., Li, Y. et al. A fusion protein strategy for soluble expression of Stevia glycosyltransferase UGT76G1 in Escherichia coli . 3 Biotech 7, 356 (2017). https://doi.org/10.1007/s13205-017-0943-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0943-y