Abstract
Stenotrophomonas maltophilia OG2 was isolated from the intestine of cockroaches that was collected from a cow barn contaminated some pesticides belong to pyrethroid and organochlorine groups. OG2 was able to degrade α-endosulfan in non sulfur medium (NSM) as a sole sulfur source for growth within 10 days of incubation. The effects of some growth parameters on endosulfan biodegradation by OG2 was studied and found that the biodegradation was significantly affected by the endosulfan concentrations, pH and temperature. Experimental results obtained in different conditions show that the optimum concentration of α-endosulfan, pH and temperature were 100 mg/L, 8.0 and 30 °C, respectively. Under these conditions, the bacterium degraded 81.53% of the α-endosulfan after 10 days. The concentration of α-endosulfan and its metabolites was determined by HPLC. Endosulfan ether, endosulfan lactone and endosulfan diol were the main metabolites in culture, but did not produce toxic metabolite, endosulfan sulfate. These results suggested that S. maltophilia OG2 degrades α-endosulfan via a hydrolysis pathway. The present study indicates that strain OG2 may have potential use in the biodegradation of pesticides contaminated environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pesticides are a class of biocide released intentionally into environment to kill living materials such as weeds (herbicides), insects (insecticides), fungus (fungicides), rodents (rodenticides) and others. They are used almost everywhere, not only in agricultural fields, but also in homes, parks, schools, buildings, forests and roads. Recent years use of synthetic pesticides in agriculture is the most widespread method for pest control. It was reported that farmers spend approximately $40 billion on pesticides annually. The main chemical groups of pesticides are organophosphate pesticides, carbamate pesticides, organochlorinated pesticides and pyretroid pesticides (Popp et al. 2013; Verma et al. 2014). Organochlorinated pesticides (DDT, endosulfan, lindane, aldrin, and dieldrin) are highly toxic and considered a potential risk to both living organisms and the environment. Endosulfan (1,4,5,6,7,7-hexachloro-5-norbornene-2, 3-dimethanol cyclic sulfite) is a chlorinated cyclodiene insecticide which is used extensively throughout the world for the control of numerous pests on a wide range of crops. The intensive use of endosulfan affects public health, beneficial biota and contaminate ecosystem (Arshad et al. 2008; Kataoka and Takagi 2013).
Technical endosulfan is a mixture of two stereoisomers, α and β-endosulfan in a ratio of 7:3. α-Endosulfan is more bioaccumulative and about three times more toxic than β-endosulfan (Negro et al. 2012). Previous studies have shown that microbial species prefer β-endosulfan for degradation over α-endosulfan (Siddique et al. 2003; Goswami and Singh 2009). Biodegradation of endosulfan and its isomers by bacteria and fungi have been previously reported (Singh and Singh 2011; Kong et al. 2013; Silambarasan and Abraham 2013; Kumar et al. 2014; Ozdal et al. 2016a). These microorganisms can utilize endosulfan either as carbon or sulfur source or both (Siddique et al. 2003). Although endosulfan is biodegradable in nature, its residues are found in environment (Weber et al. 2010). Biodegradation is affected by a number of physicochemical environmental parameters such as energy source (electron donors), nutrients, pH, temperature, and substrate concentration (Hussain et al. 2009; Singh and Singh 2011; Kong et al. 2013).
There are reports on many microorganisms capable of degrading endosulfan through the action of different of enzymes such as hydrolase, dehydrogenase, lactonase, and monooxygenase (Kataoka and Takagi 2013; Thangadurai and Suresh 2014). Endosulfan is degraded by attacking the sulphite group via either hydrolysis to form the less toxic endosulfan diol or oxidation to toxic endosulfan sulfate, respectively (Kataoka and Takagi 2013). One of the degradation products of endosulfan, endosulfan sulfate, has been found more toxic and more persistent than the endosulfan and its metabolites such as endosulfan diol, endosulfan ether, endosulfan lactone and endosulfan hydroxyether (Weber et al. 2010). Therefore, the isolation of microorganism/s that can degrade the α-endosulfan without the formation this toxic metabolite is very important.
Stenotrophomonas maltophilia is an aerobic, nonfermentative, Gram negative bacterium. S. maltophilia can be found everywhere in nature and is often used for biodegradation studies. Several reports show that S. maltophilia can degrade different compounds, such as the keratin (Bhange et al. 2016), alkane (Hassanshahian et al. 2013), toluene, benzene, ethylbenzene, xylene (Lee et al. 2002), and pesticides (Gur et al. 2014, Ozdal et al. 2016b), and phenol (Basak et al. 2014). S. maltophilia OG2 was isolated from the microflora of cockroaches living in pesticide-contaminated environment. Our previous preliminary study with S. maltophilia OG2 indicated that this strain could utilize α-cypermethrin (Gur et al. 2014). In this study, biodegradation of α-endosulfan by S. maltophilia and the factors (such as pesticide concentration, pH and temperature) effecting degradation potential were investigated for the first time.
Materials and methods
Microorganism
Stenotrophomonas maltophilia OG2 (GenBank with the accession number KC453991) was isolated during our previous study from the cockroaches (Blatta orientalis Linnaeus 1758; Dictyoptera) which live in stables contaminated with pesticides (Gur et al. 2014).
Chemicals
α-Endosulfan (C9H6Cl6O3S), endosulfan sulfate (C9H8Cl4S), endosulfan diol (C9H8Cl6O), endosulfan ether (C9H6ClO2), endosulfan hydroxyether (C9H4ClO2) and endosulfan lactone (C9H4Cl6O2) were purchased from Sigma-Aldrich (St. Louis, MO). The stock α-endosulfan solution was prepared in acetone and used for all the experiments.
Biodegradation of α-endosulfan in aqueous medium
Non sulfur medium (NSM) was used for biodegradation studies. The NSM contained (g/L): K2HPO4, 0.225; KH2PO4, 0.225; NH4Cl, 0.225; MgCl2.6H2O, 0.845; CaCO3, 0.005; FeCl2.4H2O, 0.005; glucose, 1 and 1 mL trace element solution. Trace element solution contained (mg/L): MnCl2·4H2O, 198; ZnCl2, 136; CuCl2·2H2O, 171; CoCl2·6H2O, 24; and NiCl2·6H2O, 24 (Siddique et al. 2003). The NSM was autoclaved (121 °C for 20 min), after cooling, NSM was supplemented with α-endosulfan.
Biodegradation assays
Stenotrophomonas maltophilia OG2 was grown in Nutrient Broth (Merck) aerobically at 30 °C at 150 rpm for 18 h. Cells were obtained by centrifugation (5000 rpm for 6 min at 4 °C), washed two times with sterile saline water (0.9%) and diluted with deionized water to a uniform optical density (O.D600) of 0.80. 1 mL of bacterial suspension was aseptically transferred to 50 mL sterile NSM in 250 mL Erlenmeyer flasks containing α-endosulfan and incubated on a rotary shaker. To obtain the optimal condition for α-endosulfan degradation by strain OG2, initial α-endosulfan concentration, the culture pH and temperature of medium were examined. The effect of the different initial substrate concentrations (25, 50, 100 and 200 mg/L) on the α-endosulfan degradation by S. maltophilia OG2 was investigated. The flasks were shaken at 150 rpm at 30 °C for 10 days and a pH of 7.0. To investigate the optimum pH for α- endosulfan biodegradation, the initial pH value of non sulfur medium was adjusted to pH 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 using 0.5 N HCl and 0.5 N NaOH. To investigate the optimum temperature for α-endosulfan biodegradation, cultures were incubated at 25, 30, 35 and 40 °C.
Analytical methods
α-Endosulfan and its degradation products, i.e., endosulfan sulfate, endosulfan diol, endosulfan ether and endosulfan lactone were measured by HPLC. α-Endosulfan in cultures was extracted using ethyl acetate. The sample was dried with anhydrous Na2SO4, and concentrated with a rotary evaporator. Samples were detected by HPLC using ODS C18 Hypersil Column (250 × 4.6 mm, 5 µm) as the stationary phase and acetonitrile: water (70:30, v/v) as the mobile phase. The solutes were determined utilizing UV–VIS detector at 214 nm (Hussain et al. 2009). Retention times for α-endosulfan, endosulfan sulfate, endosulfan ether, endosulfan lactone and endosulfan diol under analytical conditions were 5.323, 7.873, 8.976, 10.035 and 12.086 min, respectively. Growth was recorded spectrophotometrically at 600 nm. All experiments were conducted in triplicates.
Results and discussion
Organism
An environment without microorganism is unimaginable and microorganisms are found in almost every habitat in nature. Several interactions (pathogenic, symbiotic and vectoring) can be distinguished between insects and microorganisms (Ozdal et al. 2012; Okay et al. 2013). Many researchers have reported that pesticide degrading microorganisms isolated from pesticide contaminated sites (Singh and Singh 2011; Silambarasan and Abraham 2013; Ito et al. 2016). α-Cypermethrin degrading S. maltophilia OG2 was isolated by Gur et al. (2014) from cockroaches microflora which live in pesticide (cypermethrin and endosulfan) contaminated environment. Ozdal et al. (2016b) reported that α-endosulfan degrading S. maltophilia strains (FA8, SV3, SV9) were isolated from the body microflora of insects belong to Dermaptera (Forficula auricularia Linnaeus 1758) and Mantodea (Sphodromantis viridis La Greea 1950) orders.
Effect of α-endosulfan concentrations on the biodegradation and cell growth
The biodegradation of α-endosulfan and growth of strain OG2 was investigated at concentrations of α-endosulfan ranging from 25 to 200 mg/L (Fig. 1). After 10 days, 32.5, 48.3, 74.4 and 30.2% biodegradation of α-endosulfan were achieved at initial concentrations of 25, 50, 100 and 200 mg/L, respectively. Maximum bacterial growth was also obtained at an initial concentration of 100 mg/L. At the highest level of α-endosulfan (200 mg/L), growth and degradation were inhibited. This is also affirmed by earlier findings that indicated the inhibitory effect of high α-endosulfan concentrations on growth (Goswami and Singh 2009; Hussain et al. 2009).The presence of α-endosulfan (>200 mg/L) in the medium had a toxic effect on growth for Bordetella sp. B9 (Goswami and Singh 2009) and Pseudomonas aeruginosa MN2B14 (Hussain et al. 2009). Considering the bacterial growth and the degradation rate, 100 mg/L α-endosulfan concentration was used for further study. S. maltophilia OG2 was capable of releasing the sulfite group from α-endosulfan and utilize it as a source of sulfur for bacterial growth. Previous researchers have reported endosulfan as a sole sulfur source for microbial growth (Kalyani et al. 2009; Yu et al. 2012).
Effect of pH on the biodegradation and cell growth
Stenotrophomonas maltophilia OG2 was able to growth and degrade α-endosulfan over relatively wide range of pH (Fig. 2). It was found that change in pH significantly affect the biodegradation rate. Maximum biodegradation of α-endosulfan by S. maltophila OG2 was observed at an initial pH of 8.0 and minimum at an initial pH of 5.0. The biodegradation α-endosulfan ranged 45.5–81.53% as the broth pH increased from 5.0 to 8.0. Biodegradation was inhibited effectively at acidic pH values. At pH higher than 8, growth of S. maltophilia OG2 was slightly inhibited, which resulted to less degradation of α-endosulfan present in the culture. More biodegradation values of endosulfan at higher pH have also been reported by earlier workers (Hussain et al. 2007; Arshad et al. 2008). The slightly alkaline pH range favored the growth of this bacterial strain and the highest O.D600 (0.93) was observed in liquid culture at an initial pH of 8.0. However, the lowest O.D600 (0.32) was noted at an initial pH of 5.0 after 10 days of incubation. From the obtained result it was observed that pH of the medium has a significant effect on the growth of the microorganism and ultimately the degradation of the α-endosulfan. In general, most bacterial cultures prefer neutral to slightly alkaline conditions rather than acidic conditions for bacterial growth and so maximum degradation was in slightly alkaline pH.
Effect of temperature on the biodegradation and cell growth
Stenotrophomonas maltophilia OG2 degraded appreciable amounts of α-endosulfan in the liquid culture at all incubation temperatures (25, 30, 35 and 40 °C) for 10 days of incubation. Optimal temperature used for biodegradation was found to be 30 °C (Fig. 3). The maximum percentage of degradation was found to be 81.53, 75.6 and 70.2 in the temperature range of 30, 35, and 25 °C, respectively. Further increase in incubation temperature to 40 °C decreased the degradation of α-endosulfan. The highest O.D600, 0.93, after 10 days of incubation, was noted in the liquid culture at 30 °C followed by 0.8 and 0.84 at incubation temperatures of 25 and 35 °C, respectively. The results showed that 30 °C was the most favorable temperature for bacterial growth. Better biodegradation of endosulfan at this temperature have also been described by earlier workers (Hussain et al. 2007; Arshad et al. 2008; Yu et al. 2012). All these reports suggest that 30 °C could be the optimum temperature for the activity of the enzymes that are involved in the α-endosulfan biodegradation.
Formation of α-endosulfan metabolites by the cultures
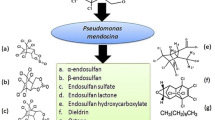
At regular intervals, the degradation of α-endosulfan and its metabolites (endosulfan diol, hydroxyether and lactone) were analysed using HPLC. As seen in Fig. 4, the rate of biodegradation products increased in NSM with time. S. maltophilia OG2 degraded about 14.71, 62.79, 81.53% of α-endosulfan in 3, 5, 10 days of incubation, respectively. α-endosulfan degradation was about 17.9% in uninoculated flasks (control) at 10th day of incubation. As seen in Fig. 4, endosulfan diol, endosulfan ether and endosulfan lactone were intermediates in the biodegradation of α-endosulfan by S. maltophilia OG2. Endosulfan ether was the major metabolite in the medium and the endosulfan sulfate was not found at any day.
Microbial degradation of endosulfan often results in the formation of a toxic endosulfan sulfate via oxidation and a less toxic endosulfan diol by hydrolysis. Endosulfan diol can further be transformed by microorganisms to endosulfan ether, endosulfan hydroxyether, endosulfan dialdehyde, and endosulfan lactone. However, these metabolites are less toxic than endosulfan and endosulfan sulfate (Kataoka and Takagi 2013; Thangadurai and Suresh 2014; Kumar et al. 2014). Several soil fungi (Botryosphaeria laricina and Aspergillus tamarii) have been shown to degrade endosulfan and produce endosulfan sulfate as the metabolic product (Silambarasan and Abraham 2013).
An initial hydrolysis of α-endosulfan results in the formation of the intermediate metabolite of endosulfan diol, than endosulfan diol was converted to endosulfan ether and endosulfan lactone (Kong et al. 2013). These results implied that OG2 degrades α-endosulfan via hydrolysis (non-oxidative) pathway (Fig. 5). Formation of these metabolites during α-endosulfan degradation has also been reported previously (Weir et al. 2006; Bajaj et al. 2010; Jesitha et al. 2015). Bajaj et al. (2010) reported that after 9 days of incubation; Pseudomonas sp. strain IITR01 was able to degrade 86% of α-endosulfan. This strain degraded α-endosulfan to endosulfan sulfate, endosulfan diol, endosulfan ether and endosulfan lactone. Goswami and Singh (2009) reported the isolation of a Bordetella sp. B9 that degraded 80% of α-endosulfan in 18 days with endosulfan lactone and endosulfan ether as intermediates. Pseudomonas sp. KS-2P (Lee et al. 2006), Achromobacter xylosoxidans C8B (Singh and Singh 2011), S. maltophilia and Rhodococcus erythropolis, and S. maltophilia E4 (Kumar et al. 2007) degraded 49% (7 days), 94.1% (20 days), 73% (14 days) and 46% (14 days) of α-endosulfan, respectively. Recently, endosulfan degrading ability of Pseudomonas fluorescens and P. aeruginosa immobilised in Ca-alginate beads were investigated by Jesitha et al. (2015) and Pradeep and Subbaiah (2016). In their studies, endosulfan diol, endosulfan ether and endosulfan lactone were detected as hydroxylated products, which indicated that these Pseudomonas species might degrade endosulfan by a non-oxidative pathway.
Different methods such as isolation of pesticide degrading microorganisms, immobilized cells (Jesitha et al. 2015; Pradeep and Subbaiah 2016), determination of pesticide degradation enzymes (Pradeep and Subbaiah 2016) and genetically engineered microorganisms (Lan et al. 2014) have been developed to reduce the effects of pesticides on the environment and health.
Conclusion
Stenotrophomonas maltophila OG2 can utilize α-endosulfan as sole sulfur source under sulfur starved condition. Several factors (temperature, pH and endosulfan concentration) can also influence the rate of biodegradation of α-endosulfan by this microorganism. The optimum endosulfan concentration, pH and temperature for maximum biodegradation ability were found to be 100 mg/L, 8 and 30 °C. The S. maltophila OG2 performed well over a fairly wide range of pH and temperature in degrading α-endosulfan in the NSM. Bacterial strain formed less toxic endosulfan diol, endosulfan lactone and endosulfan ether as metabolites during metabolism of α-endosulfan. The outcome of this research may have practical applications in biodegradation of organochlorine pesticide contaminated soil, waste dump, industrial effluents and water environments. Research should be focused on the pesticide degrading enzyme systems and the location of genes involved in degradation of the pesticide. This isolate should be immobilized using either the isolated enzymes or the whole cells for the detoxification of organochlorine and synthetic pyrethroids. Field scale studies should be conducted on the practical use of this bacterium (free or immobilize enzyme/whole cell) in cleaning up pesticides contaminate environments. Moreover, pesticide degrading aerobic and anaerobic microorganisms can be isolated from pesticide resistant insect microflora.
References
Arshad M, Hussain S, Saleem M (2008) Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa. J Appl Microbiol 104:364–370
Bajaj A, Pathak A, Mudiam MR, Mayilrajand S, Manickam N (2010) Isolation and characterization of a Pseudomonas sp. strain IITR01 capable of degrading α-endosulfan and endosulfansulfate. J Appl Microbiol 109:2135–2143
Basak SP, Sarkar P, Pal P (2014) Isolation and characterization of phenol utilizing bacteria from industrial effluent-contaminated soil and kinetic evaluation of their biodegradation potential. J Environ Sci Health, Part A 49(1):67–77
Bhange K, Chaturvedi V, Bhatt R (2016) Feather degradation potential of Stenotrophomonas maltophilia KB13 and feather protein hydrolysate (FPH) mediated reduction of hexavalent chromium. 3 Biotech 6(1):1–9
Goswami S, Singh DK (2009) Biodegradation of a and b endosulfan in broth medium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20:199–207
Gur O, Ozdal M, Algur OF (2014) Biodegradation of the synthetic pyrethroid insecticide α-cypermethrin by Stenotrophomonas maltophilia OG2. Turk J Biol 38(5):684–689
Hassanshahian M, Ahmadinejad M, Tebyanian H, Kariminik A (2013) Isolation and characterization of alkane degrading bacteria from petroleum reservoir waste water in Iran (Kerman and Tehran provenances). Mar Poll Bull 73:300–305
Hussain S, Arshad M, Saleem M, Khalid A (2007) Biodegradation of α and β-endosulfan by soil bacteria. Biodegradation 18:731–740
Hussain S, Arshad M, Shaharoona B, Saleem M, Khalid A (2009) Concentration dependent growth/non-growth linked kinetics of endosulfan biodegradation by Pseudomonas aeruginosa. World J Microbiol Biotechnol 25:853–858
Ito K, Kawashima F, Takagi K, Kataoka R, Kotake M, Kiyota H, Yamazaki K, Sakakibara F, Okada S (2016) Isolation of endosulfan sulfate-degrading Rhodococcus koreensis strain S1-1 from endosulfan contaminated soil and identification of a novel metabolite, endosulfan diol monosulfate. Biochem Biophys Res Commun 473(4):1094–1099
Jesitha K, Nimisha KM, Manjusha CM, Harikumar PS (2015) Biodegradation of endosulfan by Pseudomonas fluorescens. Environ Process 2(1):225–240
Kalyani S, Sharma S, Singh J, Dureja S, Lata P (2009) Enrichment and isolation of endosulfan-degrading microorganism from tropical acid soil. J Environ Sci Health, Part B 44(7):663–672
Kataoka R, Takagi K (2013) Biodegradability and biodegradation pathways of endosulfan and endosulfan sulphate. Appl Microbiol Biotechnol 97:3285–3292
Kong L, Zhu S, Zhu L, Xie H, Su K, Yan T, Wang J, Wang J, Wang F, Sun F (2013) Biodegradation of organochlorine pesticide endosulfan by bacterial strain Alcaligenes faecalis JBW4. J Environ Sci 25:2257–2264
Kumar K, Devi SS, Krishnamurthi K, Kanade GS, Chakrabarti T (2007) Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere 68:317–322
Kumar A, Bhoot N, Soni I, John PJ (2014) Isolation and characterization of a Bacillus subtilis strain that degrades endosulfan and endosulfan sulfate. 3 Biotechnol 4(5):467–475
Lan WS, Lu TK, Qin ZF, Shi XJ, Wang JJ, Hu YF, Chen B, Zhu YH, Liu Z (2014) Genetically modified microorganism Spingomonas paucimobilis UT26 for simultaneously degradation of methyl-parathion and γ-hexachlorocyclohexane. Ecotoxicology 23:840–850
Lee EY, Jun YS, Cho KS, Ryu HW (2002) Degradation characteristics of toluene, benzene, ethylbenzene, and xylene by Stenotrophomonas maltophilia T3-c. J Air Waste Manag Assoc 52(4):400–406
Lee JB, Sohn HY, Shin KS, Jo MS, Kim JE, Lee SW, Kwon GS (2006) Isolation of a soil bacterium capable of biodegradation and detoxification of endosulfan and endosulfan sulfate. J Agric Food Chem 54(23):8824–8828
Negro CL, Senkman LE, Vierling J, Repetti MR, García SR, Collins P (2012) Bioaccumulation in freshwater crabs. Endosulfan accumulation in different tissues of Zilchiopsis collastinensis P. (Decapoda: Trichodactylidae). Bull Environ Contam Toxicol 89(5):1000–1003
Okay S, Ozdal M, Kurbanoglu EB (2013) Characterization, antifungal activity and cell immobilization of a chitinase from Serratia marcescens MO-1. Turk J Biol 37(6):639–644
Ozdal M, Incekara U, Polat A, Gur O, Kurbanoglu EB, Tasar GE (2012) Isolation of filamentous fungi associated with two common edible aquatic insects Hydrophilus piceus and Dytiscus marginalis. J Microbiol Biotechnol Food Sci 2:95–105
Ozdal M, Ozdal OG, Algur OF (2016a) Isolation and characterization of α-endosulfan degrading bacteria from the microflora of cockroaches. Pol J Microbiol 65(1):63–68
Ozdal OG, Ozdal M, Algur OF, Sezen A (2016b) Isolation and identification of α-endosulfan degrading bacteria from insect microflora. Turk J Agric Food Sci Technol 4(4):248–254
Popp J, Peto K, Nagy J (2013) Pesticide productivity and food security. A review. Agron Sustain Dev 33(1):243–255
Pradeep V, Subbaiah UM (2016) Use of Ca-alginate immobilized Pseudomonas aeruginosa. 3 Biotech 6(2):1–13
Siddique T, Okeke BC, Arshad M, Frankenberger WT (2003) Enrichment and isolation of endosulfan degrading microorganisms. J Environ Qual 32:47–54
Silambarasan S, Abraham J (2013) Mycoremediation of endosulfan and its metabolites in aqueous medium and soil by Botryosphaeria laricina JAS6 and Aspergillus tamarii JAS9. PLoS One 8(10):e77170
Singh NS, Singh DK (2011) Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium. Biodegradation 22(5):845–857
Thangadurai P, Suresh S (2014) Biodegradation of endosulfan by soil bacterial cultures. Int Biodeterior Biodegradation 94:38–47
Verma JP, Jaiswal DK, Sagar R (2014) Pesticide relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Biotechnol 13(4):429–466
Weber J, Halsall CJ, Muir D, Teixeira C, Small J, Solomon K, Bidleman T (2010) Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci Total Environ 408(15):2966–2984
Weir KM, Sutherland TD, Horne I, Russell RJ, Oakeshott JG (2006) A single monooxygenase, ese, is involved in the metabolism of the organochlorides endosulfan and endosulfan in an Arthrobacter sp. Appl Environ Microbiol 72(5):3524–3530
Yu FB, Shinawar WA, Sun JY, Luo LP (2012) Isolation and characterization of an endosulfan degrading strain, Stenotrophomonas sp. LD-6, and its potential in soil bioremediation. Pol. J Microbiol 61(4):257–262
Acknowledgements
This research was supported by a grant from the research funds appropriated to Ataturk University (2009/236), Erzurum, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozdal, M., Ozdal, O.G., Algur, O.F. et al. Biodegradation of α-endosulfan via hydrolysis pathway by Stenotrophomonas maltophilia OG2. 3 Biotech 7, 113 (2017). https://doi.org/10.1007/s13205-017-0765-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0765-y