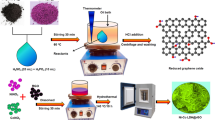
Abstract
Optimization of suitable electrode material flashing high electrochemical performance is the main hindrance in energy storage applications. Metal oxide-based electrode materials are promising candidate which greatly promotes the sustainable development. Herein, strontium oxide (SrO) is synthesized from sonochemical method followed by calcination. Incorporating polyaniline (PANI) and graphene (Gr) intensify the materials performance. Three asymmetric devices are designed using SrO, its composites (SrO/PANI and SrO/PANI/Gr) and activated carbon (AC) as an electrode and electrolyte-soaked separators are sandwiched in solidly packed cell assembly. Electrochemical measurements are performed to scrutinize the inherent properties. After that, Dunn’s model is applied to evaluate the capacitive and diffusion-controlled contributions. The obtained result divulges that SrO/PANI/Gr//AC exhibits more diffusive-controlled contribution and reveals a battery type behavior due to the contribution of PANI in redox reactions. Thus, this work delivers a route to synthesize metal oxides-based composites and a systematic approach to analyze the charge storage mechanism in asymmetric supercapacitors.











Similar content being viewed by others
References
Aguadero A et al (2012) Materials development for intermediate-temperature solid oxide electrochemical devices. J Mater Sci 47(9):3925–3948
Allen MJ, Tung VC, Kaner RB (2009) Honeycomb carbon: a review of graphene. Chem Rev 110(1):132–145
Ameen S, Shaheer Akhtar M, Husain M (2010) A review on synthesis processing, chemical and conduction properties of polyaniline and its nanocomposites. Sci Adv Mater 2(4):441–462
Ameen S et al (2011) Polyaniline/gallium doped ZnO heterostructure device via plasma enhanced polymerization technique: preparation, characterization and electrical properties. Microchim Acta 172(3–4):471–478
Ameen S et al (2012) Synthesis and electrochemical impedance properties of CdS nanoparticles decorated polyaniline nanorods. Chem Eng J 181:806–812
Ardizzone S, Fregonara G, Trasatti S (1990) “Inner” and “outer” active surface of RuO2 electrodes. Electrochim Acta 35(1):263–267
Brezesinski T et al (2010) Ordered mesoporous α-MoO 3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat Mater 9(2):146
Chen K, Xue D (2017) Colloidal paradigm in supercapattery electrode systems. Nanotechnology 29(2):024003
Chen Z et al (2012) High-performance sodium-ion pseudocapacitors based on hierarchically porous nanowire composites. ACS Nano 6(5):4319–4327
Dai L et al (2012) Carbon nanomaterials for advanced energy conversion and storage. Small 8(8):1130–1166
Di Fabio A et al (2001) Carbon-poly (3-methylthiophene) hybrid supercapacitors. J Electrochem Soc 148(8):A845–A850
Dou Y et al (2016) Atomically thin Co3O4 nanosheet-coated stainless steel mesh with enhanced capacitive Na + storage for high-performance sodium-ion batteries. 2D Mater 4(1):015022
Du Pasquier A et al (2003) A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J Power Sour 115(1):171–178
Du X et al (2008) Approaching ballistic transport in suspended graphene. Nat Nanotechnol 3(8):491
Dunn B, Kamath H, Tarascon J-M (2011) Electrical energy storage for the grid: a battery of choices. Science 334(6058):928–935
Duraisamy N et al (2016) Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J Colloid Interface Sci 471:136–144
Dylla AG, Henkelman G, Stevenson KJ (2013) Lithium insertion in nanostructured TiO2 (B) architectures. Acc Chem Res 46(5):1104–1112
Fan Q et al (2016) Activated-nitrogen-doped graphene-based aerogel composites as cathode materials for high energy density lithium-ion supercapacitor. J Electrochem Soc 163(8):A1736–A1742
Fang Q et al (2001) Ruthenium oxide film electrodes prepared at low temperatures for electrochemical capacitors. J Electrochem Soc 148(8):A833–A837
Faraji S, Ani FN (2014) Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors—a review. J Power Sour 263:338–360
Fu L et al (2019) Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: the best of both worlds? J Mater Chem A 7(25):14937–14970
Ganesh V, Pitchumani S, Lakshminarayanan V (2006) New symmetric and asymmetric supercapacitors based on high surface area porous nickel and activated carbon. J Power Sour 158(2):1523–1532
Gao Z et al (2016) A perspective on low-temperature solid oxide fuel cells. Energy Environ Sci 9(5):1602–1644
Girard H-L, Dunn B, Pilon L (2016) Simulations and interpretation of three-electrode cyclic voltammograms of pseudocapacitive electrodes. Electrochim Acta 211:420–429
Goodenough JB (2012) Evolution of strategies for modern rechargeable batteries. Acc Chem Res 46(5):1053–1061
Goodenough JB (2014) Electrochemical energy storage in a sustainable modern society. Energy Environ Sci 7(1):14–18
Hu Z et al (2015) Pyrite FeS 2 for high-rate and long-life rechargeable sodium batteries. Energy Environ Sci 8(4):1309–1316
Iqbal MZ et al (2019a) Ultrasonication-assisted synthesis of novel strontium based mixed phase structures for supercapattery devices. Ultrasonics Sonochem 104736
Iqbal MZ, Nabi J, Siddique S, Awan HTA, Haider SS, Sulman M (2019b) Role of graphene andtransition metal dichalcogenides as hole transport layer and counter electrode in solar cells. Int J Energy Res 44(3):1464–1487
Iqbal MZ, Haider SS, Siddique S, Karim MRA, Zakar S, Tayyab M, Faisal MM, Sulman M, Khan A, Baghayeri M, Kamran MA, Alherbi T, Iqbal MJ, Hussain T (2020a) Capacitiveand diffusion-controlled mechanism of strontium oxide based symmetric and asymmetric devices. J Energy Storage 27:101056
Iqbal MZ, Siddique S, Khan A, Haider SS, Khalid M (2020b) Recent developments in graphene based novel structures forefficient and durable fuel cells. Mat Res Bulletin 122:110674
Iqbal MZ, Haider SS, Zakar S, Numan A, Afzal AM, Kamran MA (2020c) Cobalt-oxide/carbon composites for asymmetric solid-state supercapacitors. Mat Res Bulletin 110974
Iqbal MZ, Zakar S, Haider SS (2020d) Role of aqueous electrolytes on the performance of electrochemical energy storage device. J Electroanal Chem 858:113793
Kalu E et al (2001) Cyclic voltammetric studies of the effects of time and temperature on the capacitance of electrochemically deposited nickel hydroxide. J Power Sour 92(1–2):163–167
Kong L et al (2016) Nanoarchitectured Nb 2 O 5 hollow, Nb 2 O 5@ carbon and NbO 2@ carbon core-shell microspheres for ultrahigh-rate intercalation pseudocapacitors. Sci Rep 6:21177
Kötz R, Carlen M (2000) Principles and applications of electrochemical capacitors. Electrochim Acta 45(15–16):2483–2498
Kou T et al (2017) Recent advances in chemical methods for activating carbon and metal oxide based electrodes for supercapacitors. J Mater Chem A 5(33):17151–17173
Kuilla T et al (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35(11):1350–1375
Laforgue A et al (2001) Hybrid supercapacitors based on activated carbons and conducting polymers. J Electrochem Soc 148(10):A1130–A1134
Larcher D, Tarascon J-M (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7(1):19
Lee C et al (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887):385–388
Li D, Kaner RB (2008) Graphene-based materials. Science 320(5880):1170–1171
Li S et al (2015) Surface capacitive contributions: towards high rate anode materials for sodium ion batteries. Nano Energy 12:224–230
Lindström H et al (1997a) Li + ion insertion in TiO2 (anatase). 1. Chronoamperometry on CVD films and nanoporous films. J Phys Chem B 101(39):7710–7716
Lindström H et al (1997b) Li + ion insertion in TiO2 (anatase). 2. Voltammetry on nanoporous films. J Phys Chem B 101(39):7717–7722
Liu T et al (2017) Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J Mater Chem A 5(34):17705–17733
Lu Q, Chen JG, Xiao JQ (2013a) Nanostructured electrodes for high-performance pseudocapacitors. Angew Chem Int Ed 52(7):1882–1889
Lu Y et al (2013b) Facile synthesis of Ni-coated Ni 2 P for supercapacitor applications. CrystEngComm 15(35):7071–7079
Lubimtsev AA et al (2013) Understanding the origin of high-rate intercalation pseudocapacitance in Nb 2 O 5 crystals. J Mater Chem A 1(47):14951–14956
Lübke M et al (2015) Highly pseudocapacitive Nb-doped TiO2 high power anodes for lithium-ion batteries. J Mater Chem A 3(45):22908–22914
Luo J-Y, Xia Y-Y (2009) Electrochemical profile of an asymmetric supercapacitor using carbon-coated LiTi2 (PO4) 3 and active carbon electrodes. J Power Sour 186(1):224–227
Mastragostino M et al (2000) Polymer selection and cell design for electric-vehicle supercapacitors. J Electrochem Soc 147(2):407–412
Morimoto T et al (1996) Electric double-layer capacitor using organic electrolyte. J Power Sour 60(2):239–247
Naor EO, Koberg M, Gedanken A (2017) Nonaqueous synthesis of SrO nanopowder and SrO/SiO2 composite and their application for biodiesel production via microwave irradiation. Renew Energy 101:493–499
Omar FS et al (2017) A promising binary nanocomposite of zinc cobaltite intercalated with polyaniline for supercapacitor and hydrazine sensor. J Alloy Compd 716:96–105
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4(4):217
Pinjari D et al (2015) Synthesis of titanium dioxide by ultrasound assisted sol–gel technique: effect of calcination and sonication time. Ultrason Sonochem 23:185–191
Prasad K et al (2010) Synthesis of titanium dioxide by ultrasound assisted sol–gel technique: effect of amplitude (power density) variation. Ultrason Sonochem 17(4):697–703
Ren B et al (2013) Hollow NiO nanofibers modified by citric acid and the performances as supercapacitor electrode. Electrochim Acta 92:197–204
Shahabuddin S et al (2019) Polyaniline-SrTiO3 nanocube based binary nanocomposite as highly stable electrode material for high performance supercapaterry. Ceram Int 45(9):11428–11437
Shanmugavalli V et al (2019) A study of charge density distribution and enhanced electrochemical properties of zinc cobaltite/polyaniline nanocomposite for supercapacitor application. Ionics 25(9):4393–4408
Simon P, Gogotsi Y (2010) Materials for electrochemical capacitors, in nanoscience and technology: a collection of reviews from nature journals. World Sci 7:320–329
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sour 196(1):1–12
Søgaard M, Hendriksen PV, Mogensen M (2007) Oxygen nonstoichiometry and transport properties of strontium substituted lanthanum ferrite. J Solid State Chem 180(4):1489–1503
Stoller MD et al (2008) Graphene-based ultracapacitors. Nano Lett 8(10):3498–3502
Thounthong P, Rael S, Davat B (2009) Energy management of fuel cell/battery/supercapacitor hybrid power source for vehicle applications. J Power Sour 193(1):376–385
Vidyadharan B et al (2014) High energy and power density asymmetric supercapacitors using electrospun cobalt oxide nanowire anode. J Power Sour 270:526–535
Wang J et al (2007) Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J Phys Chem C 111(40):14925–14931
Wang D et al (2009) Self-assembled TiO2—graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3(4):907–914
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828
Wang K et al (2014) Conducting polymer nanowire arrays for high performance supercapacitors. Small 10(1):14–31
Wang Z et al (2015) Surface modified nanocellulose fibers yield conducting polymer-based flexible supercapacitors with enhanced capacitances. ACS Nano 9(7):7563–7571
Xia X et al (2011) Three-dimentional porous nano-Ni/Co (OH) 2 nanoflake composite film: a pseudocapacitive material with superior performance. J Phys Chem C 115(45):22662–22668
Xiang C et al (2013) A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J Power Sour 226:65–70
Xiong G et al (2014) graphitic petal electrodes for all-solid-state flexible supercapacitors. Adv Energy Mater 4(3):1300515
Yan C et al (2014) Graphene/polyaniline nanocomposite as electrode material for membrane capacitive deionization. Desalination 344:274–279
Yan L et al (2016) Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 8(16):8443–8465
Yang C et al (2017) Cr0. 5Nb24. 5O62 nanowires with high electronic conductivity for high-rate and long-life lithium-ion storage. ACS Nano 11(4):4217–4224
Zheng J, Jow T (1995) A new charge storage mechanism for electrochemical capacitors. J Electrochem Soc 142(1):L6–L8
Zhou Y et al (2010) Polyaniline/multi-walled carbon nanotube composites with core–shell structures as supercapacitor electrode materials. Electrochim Acta 55(12):3904–3908
Zhou W et al (2014) Flexible wire-like all-carbon supercapacitors based on porous core–shell carbon fibers. J Mater Chem A 2(20):7250–7255
Zuo W et al (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 4(7):1600539
Acknowledgements
This work is supported by the Higher Education Commission (HEC) of Pakistan under the National Research Program for Universities (NRPU) with project Grant no. 5544/KPK/NRPU/R&D/HEC/2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iqbal, M.Z., Zakar, S., Tayyab, M. et al. Scrutinizing the charge storage mechanism in SrO based composites for asymmetric supercapacitors by diffusion-controlled process. Appl Nanosci 10, 3999–4011 (2020). https://doi.org/10.1007/s13204-020-01542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01542-4