Abstract
Boron carbide: A traditional ceramic material shows unique properties when explored in nano-range. Specially designed boron-based nanocomposite has been synthesized by reflux method. The addition of SnO2 in base matrix increases the defect states in boron carbide and shows unique catalytic properties. The calculated texture coefficient and Nelson–Riley factor show that the synthesized nanocomposite has large number of defect states. Also this composite is explored for the first time for catalysis degradation of industrial used dyes. The degradation analysis of industrial pollutants such as Novacron red Huntsman (NRH) and methylene blue (MB) dye reveals that the composite is an efficient catalyst. Degradation study shows that 1 g/L catalyst concentration of B4C/SnO2 degrades NRH and MB dye up to approximately 97.38 and 79.41%, respectively, in 20 min under sunlight irradiation. This water-insoluble catalyst can be recovered and reused.
Similar content being viewed by others
Introduction
Dyes and pigments are extensively used in textile industries, food technology, cosmetics, paper technology, leather and plastic industries for coloring the products. The estimated production of different dyes is about approximately 0.7–7 million tons per year (Christie 2007; Hunger 2003; Husain 2006). The extent of pollution caused by discharge of dyes into the environment is unknown. Use of dyes is the main source of environmental pollution and also becomes a big threat to aquatic life. The removal of dye from wastewater is a crucial need of time for better sustainability, as most of the dyes and their secondary products are carcinogenic or mutagenic and noxious in nature (Edison et al. 2016; Mady et al. 2017).
The various methods were used by researchers to decolourize different dyeing effluents. The industrial wastewater treatment involved different processes such as physical (adsorption) (Kurniawan et al. 2006; Björklund and Li 2017), chemical (ozonation) (Ikhlaq et al. 2014), biological (Shoener et al. 2014; Zhao and Liu 2016; Norman and Earnshaw 1997) reverse osmosis as well as photocatalysis (Kaur et al. 2016). The high cost of physical as well as chemical methods has failed to treat wastewater, as this comes to be economically unrealistic and also results in unavoidable secondary pollution. The photocatalysis technology is economically viable method used for degradation of dyes at ambient conditions.
Carbides have attracted attention of researchers in recent years due to their extraordinary properties such as high mechanical strength, high melting point and their chemical inertness owing to their potential applications in thermionic electron sources. Nanostructured carbides have been used in various fields such as biomaterials, light weight/high strength materials, high temperature resistant materials, and semi-conducting devices (Jia and Fischer 1996; Chen et al. 2004).
Various synthesis methods have been employed in the synthesis of boron carbide (B4C) nanostructures such as carbothermal method from reduction of boron oxide (B2O3) over 1000 ℃, thermal decomposition method, gaseous reaction between boron trichloride (BCl3) and a methane hydrogen mixture in the presence of radio frequency argon plasma, reduction of BCl3 by CH4 at 1500 ℃ with laser (Najafi et al. 2012; Wang et al. 2010). A wide range of high temperature synthesis methods can be used for preparation of B4C nanostructures directly from boron and carbon (Hajizamani and Alizadeh 2012). But these methods are economically not viable due to expensive precursors.
As metal oxides have attracted considerable attraction in research field due to their physical and chemical properties. Among them, tin oxide (SnO2) has a wide band gap (3.6 eV) and high thermal stability, so SnO2 is a potential candidate as a photocatalyst (Wang et al. 2006; Acevedo et al. 2015). The barriers in photocatalytic efficiency in case of SnO2 nanoparticles are aggregation and electron hole recombination process.
Transition of metal oxides, phosphides, and sulfides replaced high cost noble metals-based electro-catalysts as well as photo-catalysts in the past years due to their low cost and high activity (Zou and Zhang 2015). Though, corrosion and passivation under acidic conditions cause main hurdle for most of these materials. Besides, there is need of developing a stable and catalytically active material for photocatalysis process in order to reduce water pollution. Transition metal oxides usually showed their failures in the field of active site engineering. In recent years, the catalysts that contain non-metallic nature and earth abundance such as carbon are employed as alternative catalyst materials for water purification process.
Also, B4C is a semiconductor with band gap of about 1.5 eV (Domnich et al. 2011). The composite of SnO2 with B4C as base matrix can show remarkable photocatalytic properties. Also the catalyst can be recovered and reused.
The present study deals with synthesis and photocatalytic properties of B4C/SnO2 nanocomposite. B4C/SnO2 composite has been synthesized using wet chemical synthesis method in order to obtain the improved photocatalytic efficiency for wastewater treatment. Industrial pollutants, methylene blue (MB) and Novacron red Huntsman (NRH) dyes, were used as target materials. Their degradation analysis is thoroughly studied and a proposed degradation mechanism in the light of crystal structure is also presented.
Experimental section
Materials
Boric acid (H3BO3, 99.9%), activated magnesium (Mg, 98%), acetone (used as carbon source), SnCl2, and hydrochloric acid were purchased from Sigma-Aldrich. All the chemicals were used as received without any further purification.
Synthesis of B4C/SnO2 catalyst
B4C nanoparticles were successfully synthesized using solvothermal method (Singh et al. 2014, 2016). The B4C/SnO2 photocatalyst was prepared by reflux method. The freshly prepared aqueous solutions of SnCl2, B4C and HCl were added to magnetically stirred round bottom flask, respectively, and refluxed at 100 ℃ for 5 h. The obtained product was cooled to room temperature. The as-prepared sample was collected and washed with distilled water so that neutral pH is obtained. The washed precipitates were collected and dried in vacuum at 80 ℃ for 6 h.
Characterization
The dried powder of B4C/SnO2 composite was characterized by powder X-ray diffraction (XRD). The XRD pattern with diffraction intensity versus 2θ was recorded in a Rigaku instrument with Cu-Kα radiation (λ = 1.5418 Å). Transmission Electron Microscope (TEM) was carried out on TECNAI G2 20 FEI at 200 keV in order to study the morphology of synthesized material. Optical absorption spectrums were studied using UV–visible Shimadzu UV-2600 spectrophotometer.
Photocatalysis experiment
The photocatalytic activities of B4C/SnO2 (1 g/L) were evaluated by degradation of aqueous solutions of MB (1 mg/L) dye and a textile dye NRH (1 mg/L). All experiments were carried out at room temperature. The aqueous solutions were magnetically stirred for 30 min in dark to get the adsorption–desorption equilibrium followed by sunlight irradiation. The maximum absorption wavelength of MB was observed at 664 nm. Typically, 20 mg of photocatalyst (1 g/L) was added into 20 mL of 1 mg/L MB and NRH aqueous solution. Analytical samples were taken from reaction systems after specified time period and centrifuged to separate photocatalyst before analysis. The changes in absorption intensity in spectra were studied using UV–visible spectrophotometer. Similar processes were repeated for 0, 0.2 and 0.6 g/L photocatalyst dosages.
Result and discussion
XRD analysis
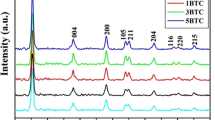
XRD pattern of the synthesized B4C/SnO2 composite is shown in Fig. 1a. The collected pattern was compared with B4C and SnO2 JCPDS cards, i.e., 35–0798 and 41–1445, respectively. The different diffraction peaks arise from B4C and SnO2. XRD analysis confirmed the formation of B4C and SnO2 phase in the synthesized sample. The broadening of diffraction peaks was used for the determination of crystallite size. The crystallite size was calculated using Debye–Scherrer formula (Cullity 1978). The calculated average crystallite size of synthesized material is equal to approximately 26 nm.
Further, XRD pattern was used to determine the texture coefficient for synthesized composite. Texture coefficient provides information about preferred growth orientation of the material. The higher the value of texture coefficient of a plane deviated from unit value, the more will be the growth along that particular plane. For the calculation of texture coefficient, standard intensities related to the crystallographic planes were taken from the standard JCPDS cards (35–0798 and 41–1445). Texture coefficient (Kumar et al. 2017, 2015) is calculated using the following relationship:
where TC(hkl), I(hkl) and I0(hkl) is texture coefficient, experimental and standard intensities of the plane specified by miller indices, respectively. The value of n represents the number of different peaks.
The texture coefficient analysis reveals that the synthesized material is more grown along (211) with a texture coefficient value 4.513. The growth of the synthesized composite along (211) can be ascribed to the presence of defects in the synthesized sample. Further, the presence of defect states has also been studied from XRD results. The variation of Δd/d with Nelson–Riley factor (Kumar et al. 2015; Singh et al. 2017) is shown in Fig. 1b. The standard values of d spacing of B4C were taken from ICDD card no. 35-0798. It is observed that in case of composite, the scatteredness of Δd/d increased. The scatteredness of Δd/d indicated the large density of defect states present in the samples (Kaur et al. 2007). Therefore, it can be concluded that the SnO2 incorporation leads to an increase in the defect state density. The effect of defect states or stacking faults in dye degradation has been discussed at the end of this section.
TEM analysis
TEM image of the as synthesized B4C/SnO2 is shown in Fig. 2. TEM image revealed that the average size of the synthesized particles is ~ 30 nm. Synthesized nanostructures are nearly spherical and agglomerated in nature. The agglomeration of particles can be ascribed to small crystallite sizes.
Mechanism followed
The presence of structural defects and distortion in B4C/SnO2 influences its structure. These inherent structural defects result in B4C/SnO2 with high efficiency in sunlight harvesting and make it a good catalyst for industrial pollutants. Existence of defects causes the downshift in conduction band and available new mid gap states that enable the boron carbide as visible light harvesting material (Liu et al. 2013).
The defects have also shown their impact on carrier relaxation dynamics, resulted in charge separation by trapping electron and holes (Joshi et al. 2010; Klimov et al. 1999; Zhang 2000). Nelson–riley plot as ell as texture coefficient indicates the presence of structural defects in B4C/SnO2. SnO2 can absorb UV wavelength light and results in electron hole separation. SnO2 makes electron availability to conduction band of B4C. These electrons and holes help to produce the ●OH radicals resulting in degradation of dyes.
Photocatalysis analysis
The synthesized B4C/SnO2 composite, employed as a photocatalyst for the degradation of MB and NRH in water under sunlight irradiation. The effect of concentration loadings of B4C/SnO2 catalyst on degradation of dyes was studied. The degradation efficiency of a dye was calculated from change in the concentration of MB and NRH dye using the following formula:
where C0 and C t were the concentrations of dye before and after that reaction. Figures 3a–d and 4a–d show the absorption intensity changes of MB and NRH dyes with different concentration of catalyst (0 to 1 g/L) under sunlight irradiation for homogeneous time interval of 20 min which specifies the degradation of dye, respectively. The removal efficiency of MB and NRH with different concentration of B4C/SnO2 catalyst is described in Table 1. Basic characteristics of MB dye and a textile dye NRH are given in Table 2. Methylene blue has absorption peak at 664 nm and a shoulder at 610 nm. The intensity of both absorption bands get reduced as sunlight irradiation time increases. This indicates the decomposition or degradation of chromo-phoric group of dye into simple intermediate smaller molecular sized molecules. On the other hands, NRH dye has a strong absorption band at 524 nm as shown in Fig. 4. The absorption peak intensity declined as time of sunlight irradiation increased from 0 to 20 min (Fig. 4a–d).
Figure 5a represents the degradation efficiency of B4C/SnO2 catalyst for degradation of MB dye. The methylene blue degradation efficiency is enhanced approximately up to 79.41% for 1 g/L B4C/SnO2 catalyst under sunlight than other concentrations of B4C/SnO2 photocatalyst.
a Degradation efficiency of B4C/SnO2 catalyst with aqueous dispersion of methylene blue (MB) dye as function of irradiation time, b average degradation rate of different concentrations of catalyst B4C/SnO2 for methylene blue (MB) at uniform time interval under sunlight irradiation, c half-life dye estimation curve from C/C0 and degradation efficiency 1-C/C0 for 1 g/L of B4C/SnO2 catalyst loading in methylene blue dye
The average decolourization rate for different catalyst loading in MB dye was calculated using Eq. 3 and is presented as a bar graph in Fig. 5b.
where C and D % are the initial concentrations of dye solution and degraded dye after time t, respectively.
The average degradation rate of MB dye with 1 g/L B4C/SnO2 catalyst is calculated as ~ 5.0425% (Fig. 5b). The half-life of a dye is defined as a time at which its concentration became half which was calculated using intersection curve of C/C0 (MB concentration) and 1-C/C0 (degradation efficiency) that was estimated approximately as 8.08 min (Fig. 5c).
Degradation efficiency for NRH dye via 1 g/L B4C/SnO2 catalyst loading is estimated to be approximately 97.38% (Fig. 6a), i.e., an excellent performance of B4C/SnO2 catalyst, under sunlight irradiation. The average degradation rate via different concentration of B4C/SnO2 catalyst for NRH dye was displayed as bar graph representation in Fig. 6b. The average degradation rate of NRH dye was calculated (using Eq. 3) as 43.82%. As the concentration of catalyst increased, the decrease in half-life was observed (Kaur et al. 2017). The half-life of NRH dye was estimated as 11.81 min shown in Fig. 6c.
a Photocatalytic degradation efficiency of different concentrations of B4C/SnO2 catalyst loading to Novacron red Huntsman dye, b Average degradation rate of different concentration of catalyst B4C/SnO2 for Novacron red Huntsman dye (NRH) at uniform time interval under sunlight irradiation, c Half-life dye estimation curve from C/C0 and degradation efficiency 1-C/C0 for 1 g/L of B4C/SnO2 catalyst loading in Novacron red dye
Conclusion
B4C/SnO2 composite synthesized by simple reflux method has been employed as a catalyst for degradation of an organic and a textile dye, i.e., MB and NRH under sunlight irradiation, respectively. The composite acts as an efficient photocatalyst due to the presence of defect states for the removal of industrial pollutants that are noxious to the humans as well as marine life. The effect of concentration of composite as catalyst on degradation under sunlight irradiation was studied. For 1 g/L B4C/SnO2 catalyst, degradation efficiency of about 79.41% was achieved with MB dye under sunlight irradiation in 20 min. The catalyst B4C/SnO2 with the dosage of 1 g/L competently degrades the NRH dye (1 mg/L) with 97.38%. The unique catalytic properties of B4C/SnO2 make it an alternative material in the field of photocatalysis.
References
Acevedo MC, Stone ML, Schmidt JR, Thomas JG, Ding Q, Chang HC, Tsai ML, He JH, Jin S (2015) Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat Mater 14:1245. https://doi.org/10.1038/NMAT4410
Björklund K, Li LY (2017) Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J Environ Manag 197:490–497. https://doi.org/10.1016/j.jenvman.2017.04.011
Chen S, Wang DZ, Huang JY, Ren ZF (2004) Synthesis and characterization of boron carbide nanoparticles. Appl Phys A Mater Sci Process 79:1757–1759. https://doi.org/10.1007/s00339-004-2913-6
Christie RM (2007) Environmental aspects of textile dyeing. Woodhead, Boca Raton/Cambridge
Cullity BD (1978) Elements of X-ray Diffraction. Addison-Wesley, New York
Domnich V, Reynaud S, Haber RA, Chhowalla M (2011) Boron carbide: structure, properties, and stability under stress. J Am Ceram Soc 94(11):3605–3628. https://doi.org/10.1111/j.1551-2916.2011.04865.x
Edison TNJI, Atchudan R, Sethuraman MG, Rok Lee Yong (2016) Reductive-degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J Photochem Photobiol, B 162:604–610. https://doi.org/10.1016/j.jphotobiol.2016.07.040
Hajizamani M, Alizadeh A (2012) Deposition of a Ni3P nano-scale layer on B4C nanoparticles by simple electroless plating in an acidic bath. Appl Nanosci 2(4):417–421. https://doi.org/10.1007/s13204-011-0055-7
Hunger K (2003) Industrial dyes: chemistry, properties, applications. Wiley-VCH, Weinheim/Cambridge
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221. https://doi.org/10.1080/07388550600969936
Ikhlaq A, Brown DR, Hordern BK (2014) Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl Catal B 154–155:110–122. https://doi.org/10.1016/j.apcatb.2014.02.010
Jia K, Fischer TE (1996) Abrasion resistance of nanostructured and conventional cemented carbides. Wear 200:206–214. https://doi.org/10.1016/S0043-1648(96)07277-8
Joshi UA, Palasyuk A, Arney D, Maggard PA (2010) Semiconducting oxides to facilitate the conversion of solar energy to chemical fuels. J Phys Chem Let 1:2719–2726. https://doi.org/10.1021/jz100961d
Kaur J, Roy SC, Bhatnagar MC (2007) Highly sensitive SnO2 thin film NO2 gas sensor operating at low temperature. Sens Actuators B 123:1090–1095. https://doi.org/10.1016/j.snb.2006.11.031
Kaur G, Singh B, Singh P, Kaur M, Buttar KK, Thakur A, Bala R, Kumar M, Kumar A (2016) Preferentially grown nanostructured iron disulphide (FeS2) for removal of industrial pollutants. RSC Adv 6:99120–99128. https://doi.org/10.1039/c6ra18838a
Kaur G, Singh B, Singh P, Singh K, Thakur A, Kumar M, Bala R, Kumar A (2017) Iron disulfide (FeS2): a promising material for removal of industrial pollutants. Chem Sel 2:2166–2173. https://doi.org/10.1002/slct.201700087
Klimov VI, McBranch DW, Leatherdale CA, Bawendi MG (1999) Electron and hole relaxation pathways in semiconductor quantum dots. Phys Rev B 60(19):13740–13749. https://doi.org/10.1103/PhysRevB.60.13740
Kumar M, Kumar A, Abhyankar AC (2015a) Occurrence of non-equilibrium orthorhombic SnO2 phase and its effect in preferentially grown SnO2 nanowires for CO detection. RSC Adv 5:35704–35708. https://doi.org/10.1039/c4ra15539d
Kumar M, Singh B, Yadav P, Bhatt V, Kumar M, Singh K, Abhyankar AC, Kumar A, Yun JH (2015b) Effect of structural defects, surface roughness on sensing properties of Al doped ZnO thin films deposited by chemical spray pyrolysis technique. Ceram Int 43:3562–3568. https://doi.org/10.1016/j.ceramint.2016.11.191
Kumar V, Singh K, Kumar A, Kumar M, Singh K, Vij A, Thakur A (2017) Effect of solvent on crystallographic, morphological and optical properties of SnO2 nanoparticles. Mater Res Bull 85:202–208. https://doi.org/10.1016/j.materresbull.2016.09.020
Kurniawan TA, Chan GY, Lo WH, Babel S (2006) Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98. https://doi.org/10.1016/j.cej.2006.01.015
Liu J, Wen S, Hou Y, Zuo F, Beran GJO, Feng P (2013) Boron carbides as efficient, metal free, visible-light- responsive photocatalysts. Angew Chem Int Ed 52:1–6. https://doi.org/10.1002/anie.201209363
Mady AH, Baynosa ML, Tuma D, Shim JJ (2017) Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation. Appl Catal Environ 203:416–427. https://doi.org/10.1016/j.apcatb.2016.10.033
Najafi A, Golestani-Fard F, Rezaie HR, Ehsani N (2012) A novel route to obtain B4C nano powder via sol–gel method. Ceram Int 38:3583–3589. https://doi.org/10.1016/j.ceramint.2011.12.074
Norman GN, Earnshaw Alan (1997) Chemistry of the Elements. Butterworth-Heinemann, ISBN, p 0080379419
Shoener B, Bradley I, Cusick R, Guest J (2014) Energy positive domestic wastewater treatment: the roles of anaerobic and phototrophic technologies. Environ Sci 16:1204–1222. https://doi.org/10.1039/C3EM00711A
Singh P, Singh B, Kumar M, Kumar A (2014) One step reduction of boric acid to boron carbide nanoparticles. Ceram Int 40:15331–15334. https://doi.org/10.1016/j.ceramint.2014.06.101
Singh P, Kaur M, Singh B, Kaur G, Kumar M, Bala R, Kumar A (2016) Gap state related blue light emitting boron-carbon core shell structures. AIP Conf Proc 1728:020690. https://doi.org/10.1063/1.4946741
Singh P, Singh K, Kaur M, Kaur H, Singh B, Kaur G, Kaur M, Kumar M, Kaur K, Bala R, Kumar A (2017) Preferentially grown nanostructured MgB2C2: a new material for lightning applications. Superlattices Microstruct 103:1–8. https://doi.org/10.1016/j.spmi.2017.01.013
Wang G, Lu W, Li JH, Choi JY, Jeong Y, Choi SY, Park JB, Ryu MK, Lee K (2006) V-shaped tin oxide nanostructures featuring a broad photocurrent signal: an effective visible-light-driven photocatalyst. Small 2:1436. https://doi.org/10.1002/smll.200600216
Wang HJ, Sun FQ, Zhang Y, Li LS, Chen HY, Wu QS, Yu JC (2010) Photochemical growth of nanoporous SnO2 at the air–water interface and its high photocatalytic activity. J Mater Chem 20:5641–5645. https://doi.org/10.1039/b926930d
Zhang JZ (2000) Interfacial charge carrier dynamics of colloidal semiconductor nanoparticles. J Phys Chem B 104(31):7239–7253. https://doi.org/10.1021/jp000594s
Zhao Q, Liu Y (2016) State of the art of biological processes for coal gasification wastewater treatment. Biotechnol Adv 34:1064–1072. https://doi.org/10.1016/j.biotechadv.2016.06.005
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44:5148. https://doi.org/10.1039/c4cs00448e
Acknowledgements
This work was funded by Board of Research in Nuclear Sciences, Department of Atomic Energy (DAE), India under project no. 34/14/41/2014-BRNS. This work was also supported by DST project No. EMR/2016/002815.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, P., Kaur, G., Singh, K. et al. Specially designed B4C/SnO2 nanocomposite for photocatalysis: traditional ceramic with unique properties. Appl Nanosci 8, 1–9 (2018). https://doi.org/10.1007/s13204-018-0662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0662-7