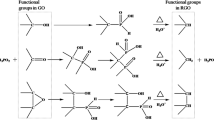
Abstract
Graphene nanosheets were produced after synthesizing graphene oxide via Hummer’s method and a modified Hummer’s method. The obtained graphene after reduction was dispersed in 1-propanol to get a coating solution. Mild steel coupons were coated with the graphene solution via dip coating method. Corrosion studies were carried out at different environments like water (pH 6.0), HCl (0.1 N), NaCl (3.5 wt%) and NaOH (1 M). Tafel analysis showed a reduction in the corrosion rate up to 99 % after three layer deposition with the graphene developed using the modified Hummer’s method. X-ray diffraction and Raman Spectroscopy confirmed the presence of graphene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corrosion is a major problem in modern societies costing nations more than billions of dollars every year (http://www.nace.org/Publications/Cost-of-Corrosion-Study/). Various approaches are undertaken for corrosion protection, protective coating being one of them. The different types of protective layers used can be in the form of metallic or alloy-based organic layers, polymeric films, paints and varnishes, oxide layers formed due to anodization etc. (Fontana 1988; Rao et al. 2009; Stratmann et al. 1994; Merkula et al. 1974; Robert et al. 2013; Pushpavanam et al. 1981). The mechanism of corrosion protection, in all the abovementioned variants, depends upon their ability to form a barrier between the active metal surface and the atmosphere (Fontana 1988). Due to difference in properties between the substrate, flaking or cracking of the coatings may occur compromising its corrosion resistant behavior (Robert et al. 2013). In some protective layers like the chromate coatings, there are serious environmental impacts concerning the disposal of the plating solutions in addition to fact of chromium being carcinogenic (Robert et al. 2013). Moreover, most of the conventionally used protective coatings result in increased thickness as well as changes in optical, electrical and thermal properties of the base material (Chen et al. 2011; Prasai et al. 2012). All these disadvantages necessitated focused research on developing thin layers of protective materials which will cause minimal changes in the properties of the protected material.
Graphene, composed of a two-dimensional hexagonal carbon network, with its extraordinary unique mechanical, optical, thermal and electrical properties has attracted a lot of interest in recent times for a wide number of applications (Prasai et al. 2012; Hu et al. 2014). Single and multilayer graphene has the potential to form ultrathin coating which does not alter the properties of the underlying material (Prasai et al. 2012; Hu et al. 2014). Graphene is considered to be inert under various atmospheres along with being impermeable to gas molecules thereby forming a natural diffusion barrier (Bunch et al. 2008). These properties make graphene a potentially important candidate for anti-corrosion films. Various researchers have studied the efficacy of graphene layers in reducing corrosion of copper and nickel substrates (Chen et al. 2011; Prasai et al. 2012; Hu et al. 2014; Bunch et al. 2008; Kirkland et al. 2012; Nayak et al. 2013; Singh et al. 2013). Graphene layers have also been grown on Aluminum (Mišković-Stanković et al. 2014) and stainless steel substrates (Pu et al. 2015). However, very scanty information is available on the impact of metal electrochemistry in presence of graphene layers. Two-dimensional graphene coatings on solid substrates are prepared by CVD, Langmuir–Blodgett method, electrophoretic deposition, electrospray etc. (Chen et al. 2011; Prasai et al. 2012; Hu et al. 2014; Bunch et al. 2008; Kirkland et al. 2012; Nayak et al. 2013; Singh et al. 2013; Mišković-Stanković et al. 2014; Pu et al. 2015) of which CVD method has emerged as the most favored one for generating graphene films. CVD synthesized graphene films have been deposited on Cu and Ni substrates and also have been transferred on to other surfaces (Prasai et al. 2012; Kirkland et al. 2012; Nayak et al. 2013; Singh et al. 2013; Mišković-Stanković et al. 2014). Elaborate and expensive experimental arrangements and precise control of temperature, concentration of precursor material and time of exposure are required to generate good quality films using CVD. However, to make use of graphene films for anti-corrosion properties more viable it is necessary to develop a low temperature, fast and effective method of film deposition so that all kinds of substrates can be easily coated.
In this work, we report a simple low temperature cost-effective method of fabricating anti-corrosion films of graphene prepared through wet chemical synthesis. Mild steel coupons were chosen as the substrate since it is the most widely used engineering material even though it has limited corrosion resistance. The graphene films were dip coated on mild steel substrates and subsequently dried in air oven at 75 °C. The process was repeated to develop multiple coating layers. The coated substrates were tested for their corrosion resistance under different environments. By being an easily corroding surface, mild steel offers an effective way of demonstrating the efficacy of graphene films as anti-corrosion layers.
Experimental methods
Graphene oxide (GO) was synthesized using both Hummer’s method (Hummers and Offeman 1958) and a modified Hummer’s method (Hirata et al. 2004). The obtained graphene oxide was subsequently reduced to obtain reduced graphene oxide (rGO). Graphite powder (Sigma-Aldrich), KMnO4 (Fisher Scientific), NaNO3 and H2O2 (30 % Analytical Reagent) (S D Fine Chem. Limited), NaBH4 (Sigma-Aldrich) were used for the synthesis of rGO. All the reagents purchased were of analytical grade. The corrosion tests were conducted on Electrochemical Analyzer/Workstation, model 700E, CH Instruments. Tafel plots were obtained using Ag/AgCl electrode as reference and Platinum wire as counter electrode. XRD study on the coating material was done using X-Ray Diffractometer (Miniflex II Rigaku) and the surface morphology was explored using Optical Microscope (STEMI 2000, Carl Zeiss). FTIR analysis was done using FTIR 8201 PC Shimadzu. Raman studies were carried out using STR250 Laser Raman system (Seki Technotron Corp., Japan).
Synthesis of GO (modified Hummers method)
Graphite powder was dried at 60 °C in an oven for 2 h to remove the moisture present in graphite powder. 0.40 g of graphite powder which was free of moisture and 0.30 g of sodium nitrate were added in a glass beaker which was placed in an ice bath. 14 ml of sulfuric acid (98 % conc.) was then added to it slowly. This mixture was under continuous magnetic stirring for 2 h. 1.8 g of potassium permanganate was added slowly to above solution while stirring in ice bath. The resulting solution was aged for 5 days. At the end of 5th day 5 wt% H2SO4 was added. After 2 h of stirring 16 ml of H2O2 was added to above solution which turned to a pale yellow color. The stirring was continued overnight and the resultant brown solution was washed with 3 wt% H2SO4 (100 ml) by centrifugation for 10 min at 10,000 rpm to obtain GO. For reduction, 13 ml of the GO solution was added with 35 ml of distilled water. Then 10 ml of 0.15 M sodium borohydride aqueous solution was added to above mixture under stirring. Temperature of above solution was maintained at 80 °C for 2 h. Final solution was washed with 100 ml distilled water by centrifugation to obtain rGO.
Synthesis of GO (Hummers method)
Two grams of graphite powder was added to 46 ml of H2SO4 under continuous magnetic stirring in an ice bath. 1 g of sodium nitrate and 6 g of potassium permanganate were added gradually and successively. The ice bath was then removed and the suspension was allowed to cool to room temperature. 92 ml of distilled water was added to this mixture. Following this, 20 ml of hydrogen peroxide was added and the solution turned bright yellow. The suspension was filtered, washed with distilled water and centrifuged to obtain GO. The synthesized GO was subsequently reduced to rGO following the same method mentioned above.
The difference between the two abovementioned synthesis techniques used was in aging allowed for 5 days in the former, which was done to ensure complete oxidation to GO. Oxidation increases the distance between the layers in graphite and improves the possibility of better separation of the graphene layers. The rGO obtained in each cased was dispersed in 1-propanol and ultra-sonicated for 1 h to obtain the coating solution.
Mild steel coupons (AISI 1010 MS, Caltech; 1 cm width and 6 cm length) were properly cleaned and dip coated at a lifting speed of 1.25 cm/s. They were subsequently dried in an air oven for 10 min at 75 °C. The graphene film adhered to the coupon by Vander Waals force. For multilayer coating, the process was repeated. Corrosion test was carried in four different environment such as Water (pH 6.0), HCl (0.1 N), NaCl (3.5 wt%) and NaOH (1 M). Ag/AgCl electrode was used as the reference, platinum as the counter electrode and the coated coupons as the working electrode. Tafel plots were obtained in each case and the corrosion rate in mils per year was determined.
Results and discussion
Graphene films have mostly been grown on copper and nickel substrates as reported in literature because they could be grown on these surfaces easily by CVD and also could be mechanically transferred onto other substrates (Prasai et al. 2012; Hu et al. 2014). However, low alloy steels form the bulk of all structural materials and due to the carcinogenic nature of chromium used in corrosion resistant coating as well as other environmental concerns, there is renewed need and opportunity of developing new coating materials and scalable technologies which will lead to efficient and sustainable approaches toward corrosion protection. In this work, we report preliminary results of the corrosion resistant behavior of graphene films on low alloy steel by a simple yet effective method.
To study the crystalline nature of the material synthesized, XRD studies were conducted on the coating material by drying the rGO solution at 75 °C for 24 h to determine the crystalline phases present. Figure 1 shows the XRD pattern obtained. A sharp peak at 2θ = 26.6° was noted. Graphene oxide has a peak centered on 2θ = 10.4° and graphene peak is centered around 2θ = 24° − 26° (Li et al. 2009; Wang et al. 2009; Mondal et al. 2012; Li et al. 2012). This is attributed to the (002) crystal plane (Li et al. 2012). The absence of any peak around 10.4° in the present case shows that the material synthesized is predominantly graphene, and the reduction of GO was mostly complete. The FTIR spectrum of the material synthesized is shown in Fig. 2. In graphene oxide peaks corresponding to C=O stretching vibration (1720–1740 cm−1) and C–O vibrations (1250 cm−1) have been reported (Marcano et al. 2010). The absence of any such FTIR peaks in the obtained spectrum signifies the transformation of GO to graphene and corroborates with the XRD results. Raman studies (Fig. 3) on the synthesized material were carried out to confirm the presence of graphene. The characteristic presence of the G and 2D peaks were noted, where the G peak is associated with the E2g phonon of C sp2 atoms and the 2D peak is the second overtone of the diamondoid D peak (Chen et al. 2011; Hu et al. 2014; Mondal et al. 2012; Tuinstra and Koenig 1970). It has been reported that the G/2D ratio varied from smaller (~0.5) to larger (~1) values for single layer to multilayer graphene films (Chen et al. 2011). In the present case, the G/2D ratio was observed to be ~0.75 which is expected since the films will have multilayer graphene. Both single and multilayer graphene coatings without having strong adhesion to the metal substrates can effectively function as inert protective layers preventing diffusion (Chen et al. 2011).
Graphene layers were developed on mild steel coupons via dip coating. Since the layers were prepared from rGO solution, incomplete coverage of surface area is a possibility. Corrosion studies were carried out on coupons coated with single and multiple layers of graphene prepared from rGO solution of GO prepared using both modified Hummer’s method (G1) and Hummer’s method (G2). To determine the optimum numbers of layers needed for good corrosion resistance multiple layers were coated on the substrate and corrosion rate was evaluated in water (pH 6.0) as a function of the number of layers deposited. Table 1 and Fig. 4 show the details of the results obtained. The bare substrate had a corrosion rate of 282 mpy (mils per year), whereas after three layers of graphene deposition the corrosion decreased to 3.5 and 22.5 mpy for the graphene obtained from G1 and G2, respectively. Since, in the modified synthesis technique 5 days of aging was allowed to improve the oxidation of graphite it most probably has resulted in graphene with less defects and better coverage, which is evidenced by the lower corrosion rates observed. A steep decrease was noted in the corrosion rate between single and double layer coating which can be attributed to a more complete coverage of the coupons with the graphene film after deposition of the second layer. With subsequent addition of layers corrosion decreased further but at a much slower rate. The corrosion rate decreased by up to 99 % with three layers of films for the graphene synthesized using G1 and 92 % for G2. All further studies were conducted on mild steel coupons coated with three layers of graphene. Figure 5 shows the characteristic Tafel plots obtained for the three layer graphene coated coupons studied for corrosion in different environments. The value for corrosion rates, obtained in each case is provided in Table 2. As can be noted in all the cases except HCl environment the corrosion rate of the coupons after three layers coating reduced to less than 10 mpy which is a significant improvement for mild steel which is easily corroded. For HCl environment, however, the corrosion rate after three layer coating was significantly higher than that observed for the other mediums. This most probably can be associated with the highly corrosive nature of the Cl− ion present in 0.1 N HCl. From the point of view of corrosion and construction materials hydrochloric acid is the most difficult to handle among common acids. Figure 6 shows the optical micrographs of the uncoated (Fig. 6a) and coated (Fig. 6b) coupons after exposure in NaCl environment for 1 h. The uncoated coupon is considerably corroded, whereas the coated coupon is almost unaffected by the environment. In most of the work reported so far where graphene has been primarily tested for its anti-corrosion properties on Cu and Ni, the corrosion of the bare substrate was much lower than mild steel in the environments tested.
Graphene layers for anti-corrosion purposes have been grown on Ni and Cu substrates mostly by CVD methods (Prasai et al. 2012; Kirkland et al. 2012; Nayak et al. 2013; Singh et al. 2013; Mišković-Stanković et al. 2014; Liang et al. 2014). Presence of graphene coatings lowered corrosion rates between 16 and 90 % (Prasai et al. 2012; Kirkland et al. 2012; Mišković-Stanković et al. 2014). The polarization curves, obtained for bare and graphene coated substrates in 0.1 M NaCl solution, showed a decrease in the corrosion current density and a shift in the corrosion potential in the positive direction, thereby confirming a decrease in the corrosion rate (Kirkland et al. 2012; Lih et al. 2012). Prasai et al. reported a decrease in corrosion rates up to 20 times for graphene coated Cu substrates in 0.1 M Na2SO4 solution (Prasai et al. 2012). Wen Pu et al. coated stainless steel (SUS304) substrates with graphene and reported a fivefold improvement in corrosion resistance from polarization tests carried out in 3.5 wt% saline environment (Pu et al. 2015). Protection from oxidation was also reported at ambient and elevated temperatures (Chen et al. 2011; Prasai et al. 2012; Kirkland et al. 2012; Nguyen et al. 2014). It has been observed that the stability of graphene layer against oxidation is dependent on its nanostructure. Defects in the structure mainly at the grain boundaries can lead to local oxidation which can be detrimental to the coatings in long term. Schriver et al. (Schriver et al. 2013) have observed that after exposures over long time scale graphene coated copper surface underwent more wet corrosion than a bare substrate. This can be attributed to the defects in the graphene layer resulting in the small anode large cathode effect, thereby increasing corrosion (Fontana 1988). Hence for sustainable anti-corrosion behavior of graphene layers, it is extremely important to have defect free proper coverage of the substrates. Various reports (Chen et al. 2011; Prasai et al. 2012; Kirkland et al. 2012; Topsakal et al. 2012; Raman et al. 2012) have mentioned the efficiency of graphene layers in protecting the underlying substrate from thermal oxidation over a short time period. However, over longer time scales this efficiency reduced and significant corrosion was noted due to the defects in the graphene layer which resulted in galvanic corrosion between graphene and Cu substrate (Hu et al. 2014). The defects also allow infiltration of oxygen and moisture toward the underlying substrate (Hu et al. 2014). Extensive researches are being carried out to develop high quality graphene films which would overcome the abovementioned problems. Graphene nanocomposite coatings of graphene nanosheets dispersed in oxide or polymer matrix may be a suitable alternative to increase the durability of these coatings and make them more robust.
Conclusion
Graphene layers are being investigated as corrosion resistant films. Most studies have been conducted on Cu and Ni substrates where films have majorly been grown by CVD. The present work details a simple and easy method of developing graphene layers on mild steel. The graphene was synthesized starting with Hummer’s and a modified Hummer’s method. Mild steel coupons were coated from the synthesized graphene solution. Three layers of graphene films were able to reduce the corrosion rate by 99 %. XRD and Raman studies confirmed the presence of graphene in the synthesized material. Further work needs to be done to test the durability of the films and its resistance to corrosion with respect to time.
References
Bunch JS, Verbridge SS, Alden JS, van der Zande AM, Parpia JM, Craighead HG, McEuen PL (2008) Impermeable atomic membranes from graphene sheets. Nano Lett 8:2458–2462
Chen S, Brown L, Levendorf M, Cai W, Ju SY, Edgeworth J, Li X, Magnuson C, Velamakanni A, Piner RR, Park J, Ruoff RS (2011) Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 5:1321–1327
Dennis RV, Viyannalage LT, Gaikwad AV, Rout TK, Banerjee S (2013) Graphene nanocomposite coatings for protecting low alloy steels from corrosion. Am Ceram Soc Bull 92(5):19–24
Fontana MG (1988) Corrosion engineering, 3rd edn. Tata McGraw-Hill Publication, New Delhi
Hirata M, Gotou T, Horiuchi S, Fujiwara M, Ohba M (2004) Thin-film particles of graphite oxide 1: high-yield synthesis and flexibility of the particles. Carbon 42:2929–2937
http://www.nace.org/Publications/Cost-of-Corrosion-Study/. Accessed Jan 2016
Hu J, Ji Y, Shi Y, Hui F, Duan H, Lanza M (2014) A review on the use of graphene as a protective coating against corrosion. Ann J Mater Sci Eng 1(3):16
Hummers W Jr, Offeman R (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kirkland NT, Schiller T, Medhekar N, Birbilis N (2012) Exploring graphene as a corrosion protection barrier. Corros Sci 56:1–4
Li Y, Tang L, Li J (2009) Preparation and electrochemical performance for methanol oxidation of pt/graphene nanocomposites. Electrochem Commun 11:846–849
Li J, Liu C, Liu Y (2012) Au/graphene hydrogel: synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J Mater Chem 22:8426–8430
Liang YQ, Yu LL, Cui ZD, Zhu SL, Li ZY, Yang XJ et al (2014) Large-scale synthetic graphene on Cu as anti-corrosion coating by chemical vapor deposition approach. Sci Adv Mater 6:545–549
Lih ETY, Ling TL, Chong KF (2012) Facile corrosion protection coating from graphene. Int J Chem Eng Appl 3(6):453
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Wei L, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806
Merkula DM, Novikov PD, Ivanenkov VN, Sapozhnikov VV, Lyakhin YI (1974) Utilization of Edn Varnish for protection of metal sea-water sampling bottles against corrosion. Oceanol Ussr 14:299–300
Mišković-Stanković V, Jevremović I, Jung Inhwa, Rhee KY (2014) Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 75:335–344
Mondal T, Bhowmick AK, Krishnamoorti R (2012) Chlorophenyl pendant decorated graphene sheet as a potential antimicrobial agent: synthesis and characterization. J Mater Chem 22:22481
Nayak PK, Hsu C-J, Wang S-C, Sung JC, Huang J-L (2013) Graphene coated Ni films: a protective coating. Thin Solid Films 529:312–316
Nguyen BS, Lin JF, Perng DC (2014) 1-nm-thick graphene tri-layer as the ultimate copper diffusion barrier. Appl Phys Lett 104:082105
Prasai D, Tuberquia JC, Harl RR, Jennings GK, Rogers BR, Bolotin KI (2012) Graphene: corrosion-inhibiting coating. ACS Nano 6:1102–1108
Pu NW, Shi GN, Liu YM, Sun X, Chang JK, Sun CL, Ger MD, Chen CY, Wang PC, Peng YY, Chia-Hung W, Lawes S (2015) Graphene grown on stainless steel as a high-performance and ecofriendly anti-corrosion coating for polymer electrolyte membrane fuel cell bipolar plates. J Power Sour 282:248–256
Pushpavanam M, Raman V, Shenoi BA (1981) Rhodium electrodeposition and applications. Surf Technol 12:351–360
Raman RKS, Banerjee PC, Lobo DE, Gullapalli H, Sumandasa M, Kumar A et al (2012) Protecting copper from electrochemical degradation by graphene coating. Carbon 50:4040–4045
Rao BVA, Iqbal MY, Sreedhar B (2009) Self-assembled monolayer of 2-(octadecylthio) benzothiazole for corrosion protection of copper. Corros Sci 51:1441–1452
Schriver M, Regan W, Gannett WJ, Zaniewski AM, Crommie MF, Zettl A et al (2013) Graphene as a long-term metal oxidation barrier: worse than nothing. ACS Nano 7:5763–5768
Singh BP, Nayak S, Nanda KK, Jena BK, Bhattacharjee S, Besra L (2013) The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 61:47–56
Stratmann M, Feser R, Leng A (1994) Corrosion protection by organic films. Electrochim Acta 39:1207–1214
Topsakal M, Sahin H, Ciraci S (2012) Graphene coatings: an efficient protection from oxidation. Phys Rev B. 85:155445
Tuinstra F, Koenig J (1970) Raman spectrum of graphite. J Chem Phys 53:1126–1130
Wang Y, Li Y, Tang L, Jin L, Li J (2009) Application of graphene-modified electrode for selective detection of dopamine. Electrochem Commun 11:889–892
Acknowledgments
Author Sai Pavan gratefully acknowledges the financial assistance received from BITS Pilani Goa Campus during his tenure as a master’s student at the Department of Chemical Engineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sai Pavan, A.S., Ramanan, S.R. A study on corrosion resistant graphene films on low alloy steel. Appl Nanosci 6, 1175–1181 (2016). https://doi.org/10.1007/s13204-016-0530-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-016-0530-2