Abstract
The photo-electrochemical properties of the layered perovskite Ba2SnO4, prepared by nitrate route are reported for the first time. The oxide exhibits a direct optical transition at 2.98 eV, attributed to the charge transfer O2−: 2p → Sn4+: 5s and possesses a chemical stability over the whole pH range. The Mott Schottky plot in acidic medium is characteristic of n type conductivity with a flat band potential Efb of −0.84 VSCE and an electron density ND of 1.14 × 1017 cm−3. The electrochemical impedance spectroscopy reveals the bulk and grain boundaries contributions. Ba2SnO4 is of interest for the environmental protection. At pH 2.5, its conduction band (CB, −0.86 VSCE) is below the HCrO4− level, yielding a spontaneous reduction to Cr3+ under UV light. The reduction is optimized with respect to the Cr(VI) concentration. The photo reduction follows a pseudo-first-order kinetic and the data fit well the Langmuir–Hinshelwood (L–H) model with an apparent rate constant of 0.18 h−1 for an initial concentration of 50 ppm. The chromate reduction under solar light is studied under optimized conditions. In presence of oxalic acid, 73 % of initial concentration disappears after ~2 h of irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environment protection has become a field of high priority because of the large scale aquatic pollution (Karunakaran et al. 2009; Gherbi et al. 2011). Unlike organic molecules, heavy metals are non-biodegradable and various methods exist for their removal, including reverse osmosis, ion exchange, electrochemical-reduction and electro-dialysis (Paul et al. 2012). However, such methods are expensive and become inefficient at low concentrations and can consequently be used as post techniques. So, the photocatalysis is a clean and cheap process and is attractive to take the relay for the water decontamination (Kaur and Pal 2012); photocatalysis has emerged as a promising solution (Hernández-Gordillo et al. 2012; Puangpetch et al. 2008). In this regard, many semiconductor (SC) oxides have been used to convert inorganic pollutants into less harmful forms, the CuFeO2-CB (~−1 VSCE) is more negative than the potential of many M2+/0 couples which can be reduced spontaneously (Nasrallah et al. 2011; Omeiri et al. 2008). The CrO42−/Cr3+ potential is too anodic with respect to CuAlO2-BC level to be involved in a direct electron transfer. So, CdS is used as mediator and the new hetero-junction CuAlO2/CdS increases the photocatalytic efficiency. Moreover, salicylic acid as hole scavenger gives the high performance among hole scavengers, and CuAlO2 approaches 100 % photostability at pH 7.5 (Brahimi et al. 2012). The applications of the photocatalysis are numerous and it is the environmental aspect that we develop through the reduction of chromate, recognized as a worldwide problem (World Health Organization International Agency for Research on Cancer 1990).
Solar energy is attractive because of its abundance, inexhaustibility and consistency. With an incident power of more than 1 kW m−2 (AM 1) at the sea level, the solar energy exceeds by far the energy the mankind needs. Chromate comes from industrial activities like tannery, batteries and microelectronic and is discharged in water at concentrations up to 500 ppm. It moves through the food chain and bio-accumulates in living organisms. The WHO has drastically limited the Cr(VI) concentration in water at 0.5 ppm and the effluents must be treated at the source.
On the other hand, a great interest has been paid to the elaboration of optically active materials, the key element in photocatalysis (Köferstein and Yakuphanoglu 2010;Noori et al. 2012). Oxides are privileged for photo-electrochemical (PEC) applications because of their chemical stability. In this respect, the stannates are expected to be good photocatalysts since they have CB of Sn-5s orbital (~4 eV below vacuum) capable to reduce any kind of inorganic ions (Omeiri et al. 2010). Comparatively, it has been reported by Fang et al. (2013) that the light-induced vapor generation at liquid-immersed metallic nanoparticles significantly enhances the photo activity. Interestingly, by increasing the incident light flux or lowering the inter-particle separation, the authors noticed the micrometer-sized bubbles resulting from the coalescence of nanoparticle-“bound” vapor envelopes.
To our knowledge, there are yet no papers on the photocatalytic activity of layered perovskites A2SnO4 (A = alkaline earth) and the PEC study has not been investigated before now. Ba2SnO4 has environmentally friendly characteristics, in addition to be low cost and chemically stable over the whole pH range (Hinatsu and Tezuka 1998). The conduction band must be positioned at energy as high as possible and barium contributes to decrease the electro affinity of the stannate. We are interesting by Ba2SnO4 because barium permits to increase the electro affinity leading to a large band bending, an attractive property for n type specimen. However, Ba2SnO4 is a low polaron semiconductor and the crystallite size plays a crucial role due to the increasing number of photocatalytic sites. Because of inherent defects in polycrystalline materials and low carrier mobility (Zidi et al. 2010), a large part of electron/hole (e−/h+) pairs are lost in the inter-grain regions, thus resulting in a poor photo activity. Therefore, we have prepared the oxide by the chemical method which has succeeded in the synthesis of homogenous powder (Wu et al. 2004) and the crystallite size becomes comparable with the diffusion length of the minority carriers. The present work deals with the chromate reduction upon UV light onto Ba2SnO4. The oxygen over-voltage is high on the stannates; the level O2/H2O is located far above the valence band and oxygen does not evolve on Ba2SnO4. So, the reduction is achieved in presence of oxalic acid as hole scavenger which promotes the charges separation and protects the oxide against photo corrosion (Lahmar et al. 2012; Iliev et al. 2010).
Experimental
Ba2SnO4 is synthesized by nitrate route. Sn (extra pure) is chemically polished with HCl (2 N), rinsed with water and dissolved in HNO3 (9 N); BaCO3 (Merck 99.9 %, dried at 250 °C) is added in stoichiometric amount and the solution is evaporated on the sand bath. The powder is ground in an agate mortar, pressed into circular discs (ϕ = 13 mm, thickness 0.5 mm) under 5 tons/cm2 and heated at 1,100 °C; the density approximates 80 %. The completion of the reaction is checked by X-ray diffraction (XRD) using Cu Kα anticathode (λ = 0.154178 nm). The diffuse reflectance spectrum is plotted with a UV–Visible–NIR spectrophotometer (Specord plus).
The point of zero charge (pzc) is determined by measuring the equilibrium pH of an aqueous solution containing a suspension of finely powdered oxide. The sample has to be n type in order to be used as photoanode and this is achieved by heating the Ba2SnO4 pellet at 600 °C under dynamic vacuum (<1 mbar). A copper wire is fixed on the back pellet with silver cement. The pellet is isolated with epoxy resin so that the outside surface (0.70 cm2) is exposed to the solution. Electrochemical measurements are conducted in the working solution (HCrO4−, pH 2.5) using a standard cell. A platinum foil serves as auxiliary electrode and all potentials are given with respect to a saturated calomel electrode (SCE) and controlled with a PGZ301 potentiostat (Radiometer). The frequency is set up at 10 kHz for the capacitance measurement. The electrochemical impedance spectroscopy (EIS) is measured at the open circuit potential (OCP = +0.236 V) by using small amplitude sine wave with a frequency analyzer in the range (10−2–105 Hz).
Glassware flasks are immersed in sulfo-chromic acid overnight and rinsed with water. The stock solution (300 ppm) is prepared from K2Cr2O7 (Merck, extra pure) in water. The concentrations down to 25 ppm are made up by dilution. The photocatalytic tests are done in a double walled Pyrex reactor containing 0.1 g of the catalyst powder in 100 cm3 of HCrO4− solution at variable concentrations, acidified by H2SO4 (pH 2.5). The chrono-potentiometric profile is recorded in the Ba2SnO4/HCrO4− suspension, the potential between the Pt electrode (Tacussel) and SCE is measured with a high impedance voltammeter (Tacussel 9A). The powder is maintained in the dark (4 h) to reach the adsorption equilibrium. The temperature is regulated at 25 °C with a thermo stated bath and the experiments are performed in air-equilibrated suspension. Then, the light is switched on, UV lamp (Phywe) serves as illumination source. The precipitate is allowed to deposit in the bottom of the reactor; the solution is centrifuged (3,000 rpm, 5 min) and the residual Cr(IV) concentration is measured with the same spectrophotometer using a 1 cm quartz cell. The efficiency of Cr(VI) photo reduction is given by:
Ao and A are, respectively, the absorbance of HCrO4− solution before and after illumination at saturation (~2 h). The solar experiments are performed on a sunny day in unstirred Pyrex reactor containing the chromate solution. The reactor is exposed to sunlight and the temperature reaches 33 °C at the end of the test. Distilled water (~0.8 MΩ cm) is used in all experiments.
Results and discussion
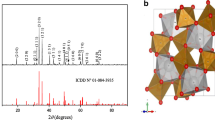
The synthesis of oxides by solid state reactions requires long time of heat treatment and high temperatures leading to irregular crystallite sizes with low active surface. By contrast, the chemical route yields homogenous powder with narrow size distribution (Čerňanský et al. 2002). The XRD pattern (Fig. 1) is characteristic of single phase; all peaks are indexed in the perovskite structure with a quadratic symmetry (SG: I4/mmm, JCPDS) in agreement with the JCPDS card N° 40–0010. The crystallite size (D ~45 nm) is evaluated from the full width at half maximum (= Kλ/β cos), where K = 0.94 is the shape factor andβ (rd) the width of the intense XRD peak (110). Assuming spherical and non-porous crystallites, a specific surface area of ~20 m2 g−1 (= 6/ρD) is obtained, the experimental density (ρ = 6.40 g cm−3) determined by hydrostatic method in toluene.
The optical properties are important in photo catalysis and the diffuse reflectance is used to get the gap (Eg); an accurate Eg value of 2.98 eV (λo = 416 nm) is obtained from the derivative curve (Fig. 2), which corresponds to the charge transfer O2−: 2p → Sn4+: 5s. The transport properties of the stannates are altered by oxygen deficiency (Trari et al. 1994). The system is electrically neutral and the formation of oxygen vacancies, generated by heating under vacuum, is achieved concomitantly with reduction of Sn4+:
The conduction occurs by electron hopping between mixed valences Sn4+/2+ accommodated in edge sharing octahedra. Ba2SnO4 is chemically stable over the whole pH range even in strong acid solutions. The intensity–potential J (V) curve in acidic medium exhibits a plateau region with a high oxygen over-voltage. The dark current is correlated to the space charge region and results from the weak electrons density (see below). The current becomes quickly large at potentials negative of ~0.4 V due to hydrogen evolution. The cathodic peak (R) and the corresponding anodic peak (O), separated by ~1.3 V are due to the internal redox process Sn4+/2+ and not to HCrO4−/Cr3+ couple since the peak persists in a chromate free solution (Fig. 3). The position of the electronic bands is determined from the variation of the interfacial capacitance with the applied potential (V):
whereεo is the permittivity of vacuum and e the electronic charge. The intercept of the straight line with the potential axis (Fig. 4) gives a flat band potential (Vfb) of −0.84 V while the positive slope lends a further support of n type conductivity. The permittivity of the material (ε–5) is determined from the dielectric measurement at 10 kHz. The linear part (C−2–V) indicates a constant density (ND) whose value (1.14 × 1017 cm−3) characterizes a lightly doped semiconductor with a wide depletion width (50 nm):
(V – Vfb ~ 0.35 V) represents the optimal band bending at the interface, required for an efficient charges separation. The lengthW extends over many crystallographic units cells and this is advantageous in photocatalysis, since an increasing number of (e−/h+) pairs are separated by the junction electric field which contribute to the photoactivity. The potential Vfb gives the energetic position of Ba2SnO4-CB with respect to vacuum:
TheP value (−0.86 V/−3.89 eV) indicates that CB is primarily made of Sn-5s orbital conferring to Ba2SnO4 a high reducing power under UV light. Ba2SnO4 is an ionic compound with a white colour and VB is made up of O2−: 2p orbital, located at −6.87 eV (P − Eg).
Photocatalysis
The solar energy continues to meet a growing demand and the photocatalysis is increasingly deployed to manage the environmental protection (Ketir et al. 2008). In view of the expensive techniques for the aquatic remediation, the exploitation of sunlight becomes highly recommended (Khataee and Kasiri 2010). Ba2SnO4 fills the photocatalytic requirements since it is non-toxic and chemically stable. However, it is responsive to UV light but the advantage resides in the high energy of the conduction band, able to reduce inorganic pollutant and the chromate reduction is used as test reaction. It is worthwhile to outline that the annual sunshine duration averages 12 h/day (Sahara) and more than 50 W m−2 of UV light is of interest for Ba2SnO4.
The stannates are small polaron oxides with low electron mobility (5.2 × 10−2 cm2 V−1 s−1).Footnote 1 Hence, the photocatalytic performance should be improved for small crystallite size (L), which must be comparable with the electron diffusion length and this can be achieved by preparing the oxide by nitrate route. The charge transfer is governed by the difference between Ba2SnO4-CB and HCrO4− level. The further advantage is that Ba2SnO4-CB varies with pH (−0.06 V pH−1) but less than HCrO4− (0.14 V pH−1) and this property has been exploited. The difference increases with decreasing pH and approaches the optimal band bending at pH 2.5, leading to an efficient separation of (e−/h+) pairs. With an acidity constant (pKa) of 1.69, H2CrO4 predominates at low pHs and converts to HCrO4− above pH 1.7. The heterogeneous transfer requires a detailed knowledge of the interfacial chemistry through the junction as well as a judicious selection of the reducing agent to prevent the holes accumulation. Oxalic acid (OA) being favourable and its mineralization occurs via holes valence band process:
The holes are consumed by OA, resulting in prolonged lifetime of carriers. The reactions occurring at the interface are the following:
The surface adsorption has a direct effect on the photoactivity, via the increasing number of the photocatalytic sites. The attachment of chromate on the catalyst powder makes easier the electron transfer. Therefore, the photocatalysis involves a dark adsorption below pzzp (= 12.7) and the enhanced activity is due simply to adsorbed HCrO4− on the catalyst powder by electrostatic forces. Since the powder cannot be polarized, the additional criterion for the photocatalysis is that the free potential (Uf = 0.041 V) must be above the potential Vfb of n-Ba2SnO4 (−0.84 V). In the dark, the potential goes to cathodic values showing that the chromate adsorption takes place onto Ba2SnO4 and saturates at ~230 mn (Fig. 5). Then, the light is switched on and the potential decreases again indicating the reduction of HCrO4− into Cr3+. It is worth mentioning that the photolysis HCrO4− (without catalyst) is weak; the concentration decreases by 2 % after 4 h irradiation and no redox reaction occurs between HCrO4− and OA Hence, the performance of Ba2SnO4 is a light-induced process. However, the moderate photoactivity is attributed to the small exchange current density (Jo ~ 200 nA/cm2) which is best though as the rate constant of electron transfer at zero potential.
The concentrations of chromate in industrial effluents can reach 300 ppm or more. Therefore, it is of interest to investigate the Cr(VI) reduction as a function of the initial concentration (Co) in the range (25–300 ppm). The results show that the reduction decreases with increasing Co (Fig. 6). At low concentrations the number of photocatalytic sites is more than the Cr(VI) ions and the photoactivity increases with raising Co until saturation of all catalytic sites by HCrO4− ion. The photons flux being constant, the chromate concentration increases and becomes greater than the number of photoelectrons, thus lowering the reduction process. The coloration of the solution also accounts for the regression in the photoactivity. Indeed, the absorption of HCrO4− solution (50 ppm) is high over the visible region; ~30 % of light is absorbed for 1 cm path at 350 nm. It is well established that the photocatalysis is efficient at low concentrations. Therefore, we have selected a concentration of 50 ppm for the further experiments (Mekatel et al. 2012).
Kinetic analysis
The kinetic of the HCrO4− reduction under illumination is given in Fig. 7. The reduction rate depends on the concentration Co and is well described by a pseudo-first-order kinetic:
the integration gives:
kapp (mn−1) is the apparent rate constant. The plot of ln (Co/C) against the illumination time (t) for different concentrations Co fits well the experimental data. The constants \( k_{\text{app}} \) and the correlation coefficients (R2) are gathered in Table 1; \( k_{\text{app}} \)decrease with increasing Co and the photo-reduction kinetic obeys to the Langmuir–Hinshelwood model (Poulios et al. 1998):
The slope and the intercept of the linear plot of k −1app versus Co (Fig. 8) give respectively the constants kr (0.193 mg L−1 min−1) and ks (23.726 L mg−1). The regression of the photoactivity may be due to the blocking of the catalytic sites by Cr(OH)3 owing to the low solubility product (Ks = 5.4 × 10−31). Indeed, the color of Ba2SnO4 powder turns to green after the photocatalytic process, due to adsorbed hydroxide.
EIS is a powerful technique to differentiate the contributions of the grain boundaries, bulk and diffusion of the whole process. The spectrum of the junction Ba2SnO4/Cr(VI) solution is measured at the open circuit potential (+0.236 V). The occurrence of two semicircles in the Nyquist plot (Fig. 9) is due to the closeness of the time constants of the bulk and grain boundaries. The centers are located below the real axis due to constant phase element (CPE). CPE represents a deviation from the ideal capacitor, defined as ZCPE = [C(jω)n]−1 where −1 ≤ n ≤ 1 andω the angular frequency. The n value may be due to the non-uniform current distribution, the roughness of the electrode and defect states within the gap region. The bulk resistance (Rb = 4.46 kΩ cm2) and grain boundaries resistance (Rgb = 6.58 kΩ cm2) are due to the lack of the reversibility of the redox couple (HCrO4−/Cr3+) over Ba2SnO4 i.e. a slow charge transfer of HCrO4−, thus corroborating the small exchange current density (Jo). The offset near the origin (1.42 kΩ cm2) is due to the electrolyte resistance (Rel). The equivalent electrical circuit is established by the Zview Software (Fig. 9, inset), the absence of the Warburg diffusion implies that the overall process is kinetically governed by the electrons flow within the space charge region of Ba2SnO4. Indeed, the electrons move with low mobility in narrow Sn-5s orbital, not exceeding 2 eV (see above).
There is a great desire to extend the chromate reduction under sunlight. The experiments are conducted under direct insulation and no correction being made from the light reflection. The light intensity varies slightly over time (Fig. 10a) and fluctuates between 90 and 103 mW cm−2 over the experiment duration. The temperature of the solution reached 33 °C at the end. The quantum yield (η) is defined as the number of HCrO4− converted per incident photonsFootnote 2 wheref(hν) is the photons flux density:
The number 3 is due to the fact that the reaction requires three electrons;η value of 0.13 % is obtained.
Despite its wide gap, the stannate shows an appreciable Cr(VI) photo reduction (Fig. 10b). However, the tendency to saturation over illumination time is ascribed to the competitive water reduction as observed in our previous work (Gherbi et al. 2011).
Conclusion
Cr(VI) is highly toxic and the ability of the perovskite Ba2SnO4 for its reduction to less harmful form is reported for the first time. Ba2SnO4 is safe, low cost and stable over the whole pH range. The nitrate route is employed for the preparation of homogeneous powder with fine particles. The oxide has been characterized structurally and photo-electrochemically. The conduction band is appropriately positioned with respect to the chromate level, allowing a spontaneous reduction under UV light. The low doping originating from small oxygen deficiency provides a large depletion layer with the occurrence of photo effect. The pH dependence of the flat band potential is judiciously exploited to have an optimal band bending at the interface. Concomitantly, the holes oxidize oxalic acid, thus inhibiting the charges accumulation. The best performance is achieved in acidic medium and the chromate follows a pseudo-first-order kinetic.
Notes
Calculated from theσ = e μeND.
\( \eta = \frac{{\int_{\text{Eg}}^{\infty } {f({h}\nu ){\kern 1pt} {\text{d}}({h}\nu )} }}{{\int_{0}^{\infty } {{h}\nu {\kern 1pt} f({h}\nu ){\kern 1pt} {\text{d}}({h}\nu ).} }} \)
References
Brahimi R, Bessekhouad Y, Nasrallah N, Trari M (2012) Visible light CrO42− reduction using the new CuAlO2/CdS hetero-system. J Hazard Mater 219:19–25
Čerňanský M, Přemysl V, Karel K, Radmila K (2002) A study of PbTiO3 crystallization in pure and composite nanopowders prepared by the sol–gel technique. Chem Monthly 133:799–806
Fang Z, Zhen YR, Neumann O, Polman A, García de Abajo FJ, Nordlander P, Halas NJ (2013) Evolution of light-induced vapor generation at a liquid-immersed metallic nano particle. Nano Lett 13:1736–1742
Gherbi R, Nasrallah N, Amrane A, Maachi R, Trari M (2011) Photocatalytic reduction of Cr(VI) on the new hetero-system CuAl2O4/TiO2. J Hazard Mater 186:1124–1130
Hernández-Gordillo A, Tzompantzi F, Gómez R (2012) Enhanced photoreduction of Cr(VI) using ZnS(en)0.5 hybrid semiconductor. Catal Commun 19:51–55
Hinatsu Y, Tezuka K (1998) Electron paramagnetic resonance study of Pr4+ions doped in BaSnO3, Ba2SnO4, and Ba3Sn2O7. J Solid State Chem 138:329–333
Iliev V, Tomova D, Rakovsky S, Eliyas A, Li Puma G (2010) Enhancement of photocatalytic oxidation of oxalic acid by gold modified WO3/TiO2 photocatalysts under UV and visible light irradiation. J Mol Catal A Chem 327:51–57
Karunakaran C, Panneerselvam Sujatha M, Mathisankar P (2009) Photoreduction of chromium(VI) on ZrO2 and ZnS surfaces. Chem Monthly 140:1269–1274
Kaur R, Pal B (2012) Size and shape dependent attachments of Au nanostructures to TiO2 for optimum reactivity of Au–TiO2 photocatalysis. J Mol Catal A Chem 355:39–43
Ketir W, Bouguelia A, Trari M (2008) Photocatalytic removal of M2+ (=Ni2+, Cu2+, Zn2+, Cd2+, Hg2+ and Ag+) over new catalyst CuCrO2. J Hazard Mater 158:257–263
Khataee AR, Kasiri MB (2010) Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes. J Mol Catal A Chem 328:8–26
Köferstein R, Yakuphanoglu F (2010) Semiconducting properties of Ge-doped BaSnO3 ceramic. J Alloys Compd 506:678–682
Lahmar H, Kebir M, Nasrallah N, Trari M (2012) Photocatalytic reduction of Cr(VI) on the new hetero-system. CuCr2O4/ZnO. J Mol Catal A Chem 353:74–79
Mekatel H, Amokrane S, Bellal B, Trari M, Nibou D (2012) Photocatalytic reduction of Cr(VI) on nanosized Fe2O3 supported on natural Algerian clay: characteristics, kinetic and thermodynamic study. Chem Eng J 200:611–618
Nasrallah N, Kebir M, Koudri Z, Trari M (2011) Photocatalytic reduction of Cr(VI) on the novel hetero-system CuFe2O4/CdS. J Hazard Mater 185:1398–1404
Noori Sepehr M, Zarrabi M, Amrane A (2012) Removal of Cr(III) from model solutions by isolated Aspergillus niger and Aspergillus oryzae living microorganisms: equilibrium and kinetic studies. J Taiwan Inst Chem Eng 43:420–427
Omeiri S, Gabes Y, Bouguelia A, Trari M (2008) Photoelectrochemical characterization of the delafossite CuFeO2: application to removal of divalent metals ions. J Electroanal Chem 6140:31–40
Omeiri S, Hadjarab B, Bouguelia A, Trari M (2010) Electrical, optical and photoelectrochemical properties of BaSnO3−δ: applications to hydrogen evolution. J Alloys Compd 505:592–597
Paul M, Pal N, Ali M, Bhaumik A (2012) New mesoporous silicotitaniumphosphate and its application in acid catalysis and adsorption of As(III/V), Cd(II) and Hg(II). J Mol Catal A Chem 330:49–55
Poulios I, Kositzi M, Kouras A (1998) Photocatalytic decomposition of triclopyr over aqueous semiconductor suspensions. J Photochem Photobio A Chem 115:175–183
Puangpetch T, Sreethawong T, Yoshikawa S, Chavadej S (2008) Synthesis and photocatalytic activity in methyl orange degradation of mesoporous-assembled SrTiO3 nanocrystals prepared by sol–gel method with the aid of structure-directing surfactant. J Mol Catal A Chem 287:70–79
Trari M, Doumerc JP, Dordor P, Pouchard M, Behr G, Krabbes G (1994) Preparation and characterization of lanthanum doped BaSnO3. J Phys Chem Solids 55:1239–1243
Wu R, Qu J, He H, Yu Y (2004) Removal of azo-dye acid red B (ARB) by adsorption and catalytic combustion using magnetic CuFe2O4 powder. Appl Catal B Environ 48:49–56
Zidi N, Omeiri S, Hadjarab B, Bouguelia A, Akroun A, Trari M (2010) Transport properties and photo electrochemical characterization of oxygen-deficient ASnO3−δ (A = Ca, Sr and Ba). Phys B 405:3355–3359
Acknowledgments
The authors are thankful to Dr M. Izzerouken for X-ray diffraction and M. Fedailine for thermal analysis. This research is financially supported by the Faculty of Chemistry (USTHB, Algiers).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Omeiri, S., Allalou, N., Rekhila, G. et al. Preparation and characterization of nano structured Ba2SnO4 as a novel photocatalyst material for the chromate reduction. Appl Nanosci 4, 959–966 (2014). https://doi.org/10.1007/s13204-013-0276-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0276-z