Abstract
The aim of this study was to formulate and characterize streptomycin-loaded PLGA-alginate nanoparticles for their potential therapeutic use in Salmonella subsp. enterica ATCC 14028 infections. The streptomycin nanoparticle was prepared by solvent diffusion method, and the other properties such as size, zeta potential, loading efficacy, release kinetics, and antimicrobial strength were evaluated. The survey shows that nanoparticles may serve as a carrier of streptomycin and may provide localized antibacterial activity in the treatment of Salmonellosis. Electron microscopy showed spherical particles with indentations. The average size of the nanoparticles was 90 nm. At pH 7.2, the release kinetics of streptomycin from the nanoparticles was successfully illustrated as an initial burst defined by a first order equation that after this stage, it has a drastic tendency to obtain steady state. Nevertheless, nanoparticles showed loading efficacy nearly about 70–75 %. In addition, the tendency of concentration of streptomycin released from nanoparticles to reach antibacterial activity was similar to that of free streptomycin against PLGA-alginate, but it had threefold more antimicrobial strength in comparison with free streptomycin. This work shows the potential use of streptomycin-loaded PLGA-alginate nanoparticles and its capability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, nanotechnology plays an important role in the most of research fields such as medicine, biology, and so on. One of the most crucial issues is drug delivery systems. In these systems, drugs have been delivered by a carrier with out any interaction between drug and capsule. These carriers are new vehicles which can cause deliver encapsulant to their right and appropriate place. Also, these systems allow protection of sensitive ingredient from unwanted reaction that lead to structural changes and emerging unpleasant taste or odor (Zohri et al. 2010). Another benefit of these systems is the control release property that can increases functional properties of food. Among different drug delivery systems, polymeric nanoparticles are good candidate for drug delivery due to their unique properties such as good stability, suitable size, and appropriately drug release (Slütter et al. 2010). Also between various carriers proposed, biodegradable nanoparticles poly(lactide-co-glycolide) are well established to have high delivery potential of manifold drugs such as alginate and chitosan that are two worthwhile polymeric materials in this area due to low cost and compatibility for vast board of materials. Also they are certified by FDA and are safe (Fathi et al. 2012). One of the most studied polymers for drug delivery is undoubtedly the poly(lactic-co-glycolic acid) (PLGA) and alginate (derivatives). These polymers have some features such as safety, biodegradability, and compact ability. PLGA has been used for controlled drug release purposes since many years ago, and it is also a suitable choice for encapsulation of antimicrobial compounds (Manca et al. 2008). Furthermore, PLGA particles are prepared by various methods; oil-in-water emulsification or solvent evaporation techniques, generally resulting in negatively charged, smooth surfaced, and spherical particles (Witschi and Doelker 1998).
These particles have properties such as resistant to salt and pH induced instability, and slowly releases their content based on the hydrolysis rate of the polymer (Sweet et al. 2007). Choi et al. (2010) used PLGA and alginate using for dual growth factor delivery system of bone morphogenetic protein (BMP)-2 and dexamethasone. Sweet et al. (2007) designed and developed a novel controlled release of PLGA alginate-pectinate polyspheric drug delivery system in 2007, and they demonstrated that as the polyspheres are provided, they are flexible yet as the superior rate-modulated drug delivery. Sivadas et al. (2008) demonstrated that a range of polymeric microspheres as potential carriers for the inhalation of proteins showed particular promise for specific protein delivery needed in the lungs.
There has been dramatically concern in recent years against the food-borne pathogen of human. Of the pathogens, there are crucial issues such as salmonella species food-borne infections constitute a significant cause of morbidity and mortality in human populations and infections caused by enterica, in particular (Zottola et al. 2012).
Streptomycin is an antibiotic medicine, which is composed of a metabolic product of Streptomyces globisporus streptomycin or another similar organism (Mashkovskii 1998). It is effective against a spectra range of bacteria and is particularly used in the treatment of tuberculosis. The major disadvantages of streptomycin are inadequate penetration into the cells due to its hydrophilicity, rapid elimination due to both efficient renal filtration, and low level of association to plasma proteins (Coessens et al. 1996).
Nurkeeva et al. (2004) produced a polycomplexes of poly(acrylic acid) with streptomycin sulfate in 2003 and evaluated their antibacterial activity using Sarcina sp. as a model organism, and they demonstrated that the polycomplexes have an antimicrobial activity on the same level as the free drug.
Doran et al. (2006) studied interpreting streptomycin susceptibility test results for Salmonella enterica serovar Typhimurium, and they found S. enterica serovar Typhimurium presenting appropriate interpretive criteria.
Hoffman et al. (1949) reported the synthesis of a macromolecular streptomycin derivative to optimize its pharmacokinetics. They attached streptomycin to methacrylic acid hydroxide and the final product was copolymerized with methacrylamide (Hoffman et al. 1949). This resulted in a non-degradable vinyl type copolymer carrier. The hydrolytic stability of the polymer-streptomycin conjugate was studied at physiological and lysosomal pH (pH 7.4 and 5.2, respectively). Streptomycin release was found to be faster in the lysosomal pH range. Despite the fact that streptomycin is an effective antimicrobial agent, there are effective factors that are mentioned before its antimicrobial potentiality might decrease, so most of the researchers also tired to keep its properties and use from their antimicrobial properties. The mentioned researchers certified that drug delivery systems were effective somewhat, but with considering type of polymers and ratio of them in formulation and even amount of encapsulant, it can be concluded that manifold products must be characterized and evaluated. In this research, new combination of streptomycin with PLGA were produced and studied.
Materials and methods
Materials include streptomycin sulfate salt, poly(d,l-lactide-co-glycolide) lactide:glycolide (50:50), mol wt 30,000–60,000, sodium alginate from Sigma-Aldrich Co was purchased. Dialysis membrane bag, 10 KD, was purchased from Spectrum Lab, USA and Salmonella subsp. enterica ATCC 14028 from Persian collection were prepared from Persian collection of bacteria in Iranian Research Organization for Science and Technology.
Method of producing the streptomycin-loaded PLGA/alginate nanoparticles
PLGA nanoparticles were prepared by a solvent diffusion method on the basis of Wang et al. (2011) with few modifications. Totally, 100 μl of streptomycin solution (100 mg/ml) was added drop-wise to alginate solution and stirred for 30 min. Then 100 mg of PLGA was added into alginate (dissolved in deionized water) surfactant solution with different concentrations (0.1, 0.2, 0.5, and 1.0 %) through a syringe pump at constant rate (20, 40, and 60 ml/h) under stirring at 200 rpm, and then nanoparticles were collected by centrifugation (Beckman Co., Avanti 30) (14,000 rpm, 15 min). The nanoparticles were resuspended using deionized water and centrifuged thrice to remove excess alginate. A fine powder of charged nanoparticles was obtained by lyophilization for 2 days.
Morphology of the nanoparticles
Scanning electron microscopy was used for shape and occurrence of aggregation phenomena evaluation. Prior to observation, 10 μl of the sample of nanoparticle suspensions were mounted on metal stub plating coated under vacuum and then for more investigation examined by scanning electron microscopy (FWH-1200A, 500 V, UK). All formulations were evaluated with scanning microscopy and formation of nanoparticles was assessed.
Size and zeta potential of the nanoparticles
Zetasizer 3000 HS (Malvern Instruments, UK) evaluated the size and surface zeta potential of the nanoparticles. Each sample was diluted (1:1) with 10 diluted aqueous dispersion of the nanoparticles prepared from the three polymer ratios and measured by the device in triplicate (Blanco and Alonso 1998).
Determination of loading efficiency of the nanoparticles
For this assessment, streptomycin-loaded nanoparticles dispersions were centrifuged using a 100-kDa molecular weight cut off ultrafilter (Amicon Ultra-15 Ultracel-100 K) at 15,000×g, 25 °C for 10 min. Then, the streptomycin loading efficiency (LE) was calculated using Eq. 1 (Zohri et al. 2011):
Release assessment of streptomycin from the nanoparticles
Release of streptomycin was counted by dialysis method. First of all, the 10 kDa (MWCO) membrane was selected as the MWCO was large enough to allow passage streptomycin to pass. For the experiment, 5 ml of a 10-mg/ml solution of phosphate-buffered saline (PBS) at pH 7.4 (release media) was poured into the inner tube of the dialyzer. The dialyzer tube was placed into a 100 ml glass cylinder containing release media, which was continually stirred at 300 rpm using a small magnetic stir bar to prevent the formation of an unstirred water layer at the membrane/outer solution interface. Diffusion to the outer solution at 37 °C was assessed by sampling the contents of the outer solution at periodic intervals of 0, 10.20, 40, 60.80, 100, 120, 150, 180, 200 (Zohri et al. 2011).
Sensitivity test against salmonella
Salmonella subsp. enterica ATCC 14028 was prepared from PTCC (Persian Type Culture Collection). Culture methods consist of inoculating 1 ml of stock into 100 ml of tetrathionate broth and incubating at 37 °C for 48 h, subculturing 10 ml of the tetrathionate broth into 100 ml RV broth, and incubating at 37 °C for 24 h, then inoculating XLT4 agar plates with 10 ml of the RV broth and incubating 24 h at 37 °C before screening for suspect Salmonella colonies. Also the microbial count for three samples as control sample which include salmonella and the sample that includes free streptomycin + Salmonella and nanoparticle + Salmonella in three replicate (Nurkeeva et al. 2004).
Testing of antibacterial activity using agar well diffusion method
Resistant bacterial strains were inoculated into 10 ml of sterile nutrient broth, and incubated at 37 °C for 8 h. Each culture was swabbed on the surface of sterile nutrient agar plate in duplicate. In each agar plate of both sets, four wells were prepared with the help of sterilized cork borer of 10 mm diameter on the basis of Palashka method (Palaksha et al. 2010). In the wells of first plate of each set, 100 μl test samples of following concentrations: (1) standard streptomycin 10 mg/ml in sterile distilled water, (2) streptomycin-loaded nanoparticles (5 %), (3) streptomycin-loaded nanoparticles (2 %), (4) streptomycin-loaded nanoparticles (1 %) were added using micropipette. Every plate used according to the aforementioned procedure was performed in triplicate for statistical average.
Statistical analysis
All of the experiments were done in triplicates and the averages of the data were compared with independent t-test. A p value of 0.05 was considered as statistical significance.
Results and discussion
Microscopic image assessment
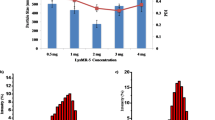
The morphology of the nanoparticles was analyzed by transmission electron microscopy (Fig. 1) and it was illustrated that the particles were separate, spherical with in the size ranging from 55 to 98 nm. The results of similar research had agreement with other researcher results (Slütter et al. 2010; Witschi and Doelker 1998). Results also showed that from four concentrations 0.5 % due to electrostatic stability and charge, balance was stable and observable under microscope (Zohri et al. 2010). In formulations 0.1, 0.2 and 1 % concentrations, nanoparticles were not formed or were not stable for observation under SEM. Figure 2 show scanning electron microscope for 0.5 % ratio. So other characterizations were done for 0.5 %.
Size and zeta potential assessment
The image illustrated that the mean size of nanoparticles were between 10 and 100 nm, and the mean of PDI (polydispersity index) of the nanoparticles was 0.30 mv. PDI is the index which indicates the homogeneity of the particles. The zeta potential of nanoparticles showed that the nanoparticles have good stability (Fig. 1).
Release profile and loading efficiency evaluation of the nanoparticles
Release of an entrapped protein from particle involves the swelling and loosening of the compact structure of the particle, allowing protein molecules entrapped at sub layers of the particles to divorce under hydrolytic actions, and then disperse through and come-off from the particle structure. There are two steps in this release process, one stage is called as burst release that include swelling of nanoparticles specially polysaccharides which happens 0–60 min after starting of release. It was found that the initial burst of release might be due to the degradation of the PLGA chain (Niwa et al. 1994), and after this stage will be release toward a steady stage for 200 min from the initial release. Loading efficiency was determined 70–75 % using the Eq. 1 because of good reaction and stability of nanoparticles, Zeta potential results were also in agreement with these results (Fig. 3).
Microbial assessment
The results showed that the samples with streptomycin-loaded PLGA/alginate nanoparticles (5 %) had zone diameter value ≥10 mm in comparison with samples with naked streptomycin, which was about maximum 7 mm and Streptomycin-loaded PLGA nanoparticles (2 %) and (1 %) with zone diameter about 3 mm (Fig. 4). Microbial count results also confirmed this (Fig. 5). Doran et al. (2006) also determined the streptomycin MIC for isolates of Escherichia coli with zone diameters of 11–12 mm, and they suggested that interpretive criteria for MIC results should be susceptible at 8 mg/l and resistant at 16 mg/l. Microbial count showed that in sample with nanoparticles, salmonella growth was completely inhibited. Maintaining streptomycin structure and helping antimicrobial strength maintenance are of crucial importance (Doran et al. 2006). Another issue is due to synergistic effects of PLGA, and it is highly affected by the streptomycin properties and its possible interaction with PLGA and its degradation products. The results of blanco et al. was in agreement with this finding (Blanco and Alonso 1998; Brenda and Rostagno 2008; Neeraj et al. 2008).
Conclusion
Interaction between streptomycin sulfate and PLGA in aqueous solutions led to the formation of good stability nanoparticles. The structure of the nanoparticles is compact and their stability/solubility is greatly dependent on the pH. Release of streptomycin is a pH-dependent factor with attention to the in vitro release results, about 90–95 % release, in the medium with pH of 7.4 (because this pH leads to the more positive charge of nisin and consequently, more partitioning of streptomycin into the release medium) (Zohri et al. 2010). It was shown that the nanoparticles have the same level of antimicrobial activity as the free drug. The information obtained in the present work can be used for the design of new drug delivery systems and can be also taken into account for the prediction of drug interactions within pharmaceutical formulations.
References
Blanco D, Alonso MJ (1998) Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm 45(3):285–294
Brenda CL, Rostagno MH (2008) Comparison of five culture methods for salmonella isolation from swine fecal samples of known infection status. J Vet Diagn 20:620–624
Choi DH, Parka CH, Kimb IH, Chunc HJ, Parka K, Hana DK (2010) Fabrication of core–shell microcapsules using PLGA and alginate for dual growth factor delivery system. J Control Release 147(2):193–201
Coessens V, Schacht E, Domurado D (1996) Synthesis of polyglutamine and dextran conjugates of streptomycin with an acid-sensitive drug- carrier linkage. J Control Release 38:141–150
Doran G, NiChulain M, DeLappe N, O’Hare C, Corbett-Feeney G, Cormican M (2006) Interpreting streptomycin susceptibility test results for Salmonella enterica serovar Typhimurium. Int J Antimicrob Agents 27(6):538–540
Fathi M, Mozafari MR, Mohebbi M (2012) Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Tech 23:13–27
Hoffman SH, Hyman GA (1949) The combined use of streptomycin and pneumoperitoneum in the treatment of pulmonary tuberculosis. Chest 15(3):354–365. doi:10.1378/chest.15.3.354
Manca ML, Spyridon M, Vassileios D, Maria FA (2008) PLGA, chitosan or chitosan-coated PLGA microparticles for alveolar delivery? A comparative study of particle stability during nebulization. Colloids Surf B Biointerfaces 62:220–231
Mashkovskii MD (1998) Lekastvennye sredstva (in Russian). Torsing, Kharkov
Neeraj S, Desmond OR, Aoife T, Vivienne B, Zeibun R, Kellya JG, Hickeyb AJ, Cryana SA (2008) A comparative study of a range of polymeric microspheres as potential carriers for the inhalation of proteins. Int J Pharm 358:159–167
Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y (1994) In vitro drug release behavior of D, L-lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate prepared by a novel spontaneous emulsification solvent diffusion method. J Pharm Sci 83(5):727–732
Nurkeeva ZS, Khutoryanskiy VV, Mun GA, Sherbakova MV, Ivaschenko AT, Aitkhozhina NA (2004) Polycomplexes of poly (acrylic acid) with streptomycin sulfate and their antibacterial activity. Eur J Pharm Biopharm 57(2):245–249
Palaksha MN, Ahmed M, Das S (2010) Antibacterial activity of garlic extract on streptomycin-resistant Staphylococcus aureus and Escherichia coli solely and in synergism with streptomycin. J Nat Sci Biol Med 1(1):12–15
Sivadas N, O’Rourke D, Tobin A, Buckley V, Ramtoola Z, Kelly JG, Hickey AJ, Cryan SA (2008) A comparative study of a range of polymeric microspheres as potential carriers for the inhalation of proteins. Int J Pharm 358(1–2):159–167
Slütter B, Bal S, Keijzer C, Mallants R, Hagenaars N, Que I, Kaijzel E, Willem VE, Patrick A, Clemens L, Joke B, Femke B, Wim J (2010) Nasal 5 vaccination with N-trimethyl chitosan and PLGA based nanoparticles: nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine 28:6282–6291
Sweet JL, Pillay V, Choonara YE (2007) Design and development of a novel controlled release PLGA alginate-pectinate polyspheric drug delivery system. Drug Deliv 14(5):309–318
Wang Q, Jamal S, Detamore MS, Berkland C (2011) PLGA-chitosan/PLGA-alginate nanoparticles blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J Biom Materials Res Part A 96(3):520–527
Witschi C, Doelker E (1998) Peptide degradation during preparation and in vitro release testing of poly (l-lactic acid) and poly(dl-lactic-co-glycolic acid) microparticles. Int J Pharm 171(1): 1–18
Zohri M, Shafiee Alavidjeh M, Haririan I, Shafiee Ardestani M, Sadat Ebrahimi SE, Tarighati Sani H, Sadjadi SK (2010) A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob Prot 2:258–266
Zohri M, Nomani A, Gazori T, Haririan I, Mirdamadi SS, Sadjadi SK, Ehsani MR (2011) Characterization of chitosan/alginate self-assembled nanoparticles as a protein carrier. J Dis Sci Tech 32(4):576–582
Zottola T, Montagnaro S, Magnapera C, Sasso S, De Martino L, Bragagnolo A, D’Amici L, Condoleo R, Pisanelli G, Iovane G, Pagnini U (2012) Prevalence and antimicrobial susceptibility of Salmonella in European wild boar (Sus scrofa); Latium Region–Italy. Comp Immunol Microbiol Infect Dis 12:00128-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Asadi, A. Streptomycin-loaded PLGA-alginate nanoparticles: preparation, characterization, and assessment. Appl Nanosci 4, 455–460 (2014). https://doi.org/10.1007/s13204-013-0219-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0219-8