Abstract
The present paper reports observations made on investigations carried out to study structural, optical and electrical properties of thermally evaporated ZnO thin films and their modulations on doping with metallic indium and indium oxide separately. ZnO thin film in the undoped state is found to have a very good conductivity of 90 Ω–1 cm–1 with an excellent transmittance of up to 90 % in the visible region. After doping with metallic indium, the conductivity of the film is found to be 580 Ω–1 cm–1, whereas the conductivity of indium oxide-doped films is increased up to 3.5 × 103 Ω–1 cm–1. Further, the optical band gap of the ZnO thin film is widened from 3.26 to 3.3 eV when doped with indium oxide and with metallic indium it decreases to 3.2 eV. There is no considerable change in the transmittance of the films after doping. All undoped and doped films were amorphous in nature with smooth and flat surface without significant modifications due to doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc oxide (ZnO) thin film has attracted much attention in the research community due to the coexistence of conductivity and transparency in the visible region of the electromagnetic radiation spectrum. A major driving force of research in zinc oxide thin film is its prospective use as thin film solar cells (Martinez et al. 1997), thin film transistors (Fortunato et al. 2005), gas sensors (Sahay and Nath 2008) and optoelectronic devices in the UV region (Moon et al. 2005).
Many methods have been employed to obtain good quality of ZnO thin films. R.F sputtering (Jeong et al. 2003), pulsed laser deposition (Craciun et al. 1994), spray pyrolysis technique (Prasad and Kumar 2009), sol–gel method (Chien et al. 2010), etc. are some of the examples. Despite the interest in ZnO thin films, not much attention has been paid toward its preparation by thermal evaporation technique. Thermal evaporation technique is a relatively simple method and is a low-cost procedure. It does not require any catalyst or high temperature growth. The temperature for further oxidation of ZnO film in this method is also moderate enough to be easily applied to thin film technology. Much of previous studies on ZnO thin films deposited by thermal evaporation technique concentrated on the effect of annealing and oxidation mechanism of films (Aly et al. 2001; Aida et al. 2006).
A great deal of activity has been focused recently on the development of doped ZnO thin films. Since zinc oxide is an n-type semiconductor, n-type doping is much easier compared to p-type doping. Doping with aluminum (Al), gallium (Ga) and indium (In) has been attempted by many groups using different deposition techniques and with different sources of dopants resulting in high-quality ZnO thin films (Sahay and Nath 2008; Prasada and Kumar 2010; Hafdallah et al. 2011; Ma et al. 2007; Tokumoto et al. 2002). The room temperature conductivity of indium-doped ZnO thin film has been already reported (Hafdallah et al. 2011; Lee et al. 1996; Lucio et al. 2006). However, there are very few reports on the study of doping of ZnO thin films using different sources of dopants.
However to the best of our knowledge, this is the first attempt to improve the electrical conductivity of ZnO thin films obtained by thermally evaporating pure ZnO powder through doping with indium (In) and indium oxide (In2O3) separately. We report the growth and characterization of ZnO thin films obtained by thermally evaporating pure ZnO powder under vacuum. Further, the conductivity of the films is improved by doping it with 5 % In and 5 % In2O3. Metallic indium and indium oxide are separately used as the source of n-type dopants. In both the cases, indium being a third group element is expected to occupy the zinc lattice site. This work is an attempt to study the nature of indium as a dopant in its pure metallic state as well as in the oxide state.
Experimental work
Undoped ZnO thin films were obtained by thermally evaporating pure ZnO powder (99.99 %, Alfa Aesar, Ward Hill, USA). These ZnO thin films were coated on clean glass slides maintained at room temperature under vacuum of the order of 10–5 torr, using tungsten as the evaporating source.
For the preparation of doped films, a mixture of 95 % of ZnO powder and 5 % of dopant is evaporated maintaining other deposition parameters the same as those of undoped condition. In the present study, metallic indium powder and indium oxide powder (99.9 %, Alfa Aesar, Ward Hill, USA) were used as the two dopants. The thickness of films obtained is measured using gravimetric method and the thickness of all film samples was found to be 200 nm.
During the process of evaporation, ZnO powder decomposes into zinc and atomic oxygen according to the kinetics of evaporation of ZnO, and oxygen will be released as the primary by-product of the process (Boris et al. 2004). Thus, films obtained are expected to be oxygen deficient in the as-deposited condition and they are found to be dark brown in color and opaque in nature. Hence, all undoped and doped films were then annealed at 300 °C for 2 h for further oxidation (Aida et al. 2006) and all films turned transparent. This is due to the re-oxidation of the film and hence the improvement in the stoichiometry of the film. Some reports have proved the improvement in stoichiometry of thermally evaporated ZnO thin films after annealing (Aly et al. 2001; Aida et al. 2006; Bouhssira et al. 2006). The annealed films were then subjected to different characterizations as follows.
Structural characterization of the obtained ZnO thin films was studied by X-ray diffractometer using JEOL diffractometer with a scanning rate of 1°/min. The scanning electron microscopy (SEM) to study the surface morphology of the films was performed with JEOL JSM 6380 system. Transmittance and absorbance measurements were carried out relative to the uncovered substrate at normal incidence in a spectral range of 250–850 nm using Ocean Optics Inc SD 2000 UV–VIS spectrometer. The electrical conductivity of the ZnO thin films was found out on coplanar structures consisting of two silver strips evaporated on the film surface and measurements were carried out with computer-assisted Keithley source meter (2400) setup.
Results and discussion
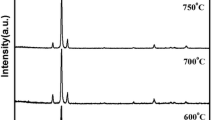
X-ray diffractogram showed that all doped and undoped films obtained were amorphous in nature in the as-deposited condition as well as after annealing. This amorphous nature leads to flat and smooth surface of the film and low internal stress; such films are well suited for the application of flat panel displays (Shigesato 2010).
The scanning electron micrographs of the surface of the undoped and doped ZnO films are shown (Figs. 1, 2, 3). It is clear from these micrographs that there is no modification in the surface topography of the films after doping. It is observed that all undoped and doped films have a smooth, continuous and pinhole-free microstructure.
The transmittance spectra of the films, taken after annealing at 300 °C for 2 h, shows an excellent transmittance of an average of 90 % in the visible region of the electromagnetic spectrum. From the spectra, it was shown that doping did not decrease the transmittance of the films and all undoped, In-doped and In2O3-doped films had almost equal transmittance in the visible region (Fig. 4).
Since ZnO is a direct band gap semiconductor allowed direct transitions can be assumed, and the spectral dependence of the absorption coefficient α can be described using Eq. (1) (Khairnar et al. 2003).
The variation of (αhν)2 with hν is plotted and the linear portion of the plot is extrapolated to zero to find the band gap of the film (Fig. 5). It is observed that the band gap of the undoped film is 3.26 eV.
When the film is doped with 5 % of In metal, the band gap is reduced to 3.2 eV. This decrease in the band gap is due to the formation of donor levels below the conduction band, which will absorb the photons having energy lower than the band gap value.
The band gap is widened to 3.3 eV when the film is doped with 5 % of In2O3. This widening of optical band gap is generally attributed to the Burstein–Moss shift (Kykyneshi et al. 2010), which results from the filling of electronic states near the bottom of the conduction band, due to the increase of carrier concentration in the ZnO film. In this process, the apparent band gap of a semiconductor is increased as the absorption edge is pushed to higher energies, because of all states close to the conduction band being populated. Tokumoto et al. (2002) also made the same observation when a ZnO thin film grown by spray pyrolysis method was doped with indium.
The I–V characteristics of undoped and doped ZnO films show the stability of current over a range of voltage from –5 to 5 V and the reduction in the resistance of the samples due to doping (Fig. 6).
The conductivity of the present films was measured in a coplanar structure obtained with evaporation of two silver stripes on the film surface.
The method is mainly based on the formula.
where σ is the conductivity of the film, A is the area between two contacts, R is the resistance of the film and l is the length of the contacts.
The area between the silver contacts can be written as the product of thickness of the film (t) and gap between the two contacts (d).
Thus, the conductivity of the film will be equal to
The room temperature conductivity of the undoped film is found to be 90 Ω–1 cm–1. This value of conductivity is found to be considerably high as compared to previously reported results on the conductivity of undoped ZnO thin films (Craciun et al. 1994; Banerjee et al. 2006; Rusu et al. 2007).
Oxygen vacancies present in the film even after the annealing process may be the major reason for this high conductivity of the film. It is well known that the conductivity of ZnO thin film can be improved by the creation of intrinsic donors by the lattice defect. Oxygen vacancies present in the film will act as intrinsic defects and are responsible for the conduction electron carrier generation in ZnO. The electron generation can be explained using Kroger–Vink notation according to Eq. (2) (Kykyneshi et al. 2010).
From Eq. (2), it is clear that when an oxygen anion escapes from the crystal structure (½O2) from the occupied oxygen site, a doubly ionized vacancy site (Vo) is created with the liberation of two electrons. Thus, multiple carriers are created from a single defect and good n-type conductivity can be obtained. Hence, low resistivity at room temperature in ZnO thin film can be achieved by the creation of intrinsic donors by lattice defects such as oxygen vacancies.
However, these intrinsically doped films will not be stable at ambient conditions. At high temperature, these films will undergo oxidation and there will be a reduction in the oxygen vacancies, leading to the significant increase in the film resistivity. Moreover, the resistivity of the intrinsically doped film can be reduced only up to 10–2–10–3 Ω cm. Thus, these intrinsically doped films are not well suited for device application (Elmer 2010).
After doping the film with 5 % of metallic indium, the conductivity of the film is increased to 580 Ω–1 cm–1. When the film is doped with 5 % of In2O3, a very good improvement in the conductivity is observed and the room temperature conductivity of the film is found to be 3.5 × 103 Ω–1 cm–1.
The high conductivity of indium oxide-doped ZnO can be explained by considering Eq. (3).
According to Eq. (3), when In2O3, which is a group III oxide, is added to ZnO, it is assumed that, being a group III dopant atom, indium will occupy the zinc lattice site spending the additional electrons which are not required for the bonding to the conduction band (Elmer 2010):
These additional electrons will contribute to improving the n-type conductivity of the film. Hence, the conductivity of the films doped with In2O3 is found to be very high as compared to that of films doped with metallic indium. The conductivity and percentage transmittance of indium-doped ZnO thin films obtained by different methods and using different dopant sources reported by other research groups are shown along with the present results in Table 1.
Conclusions
We have used thermal evaporation method as a simple and low-cost technique for the preparation of ZnO thin films. Due to the oxygen vacancy in the ZnO thin film which acts as intrinsic defect, a high conductivity of up to 90 Ω–1 cm–1 was achieved in the undoped state itself. On doping the film with metallic indium, decrease in the optical band gap was observed, whereas with indium oxide band gap widening was noticed. When the film is doped with metallic indium, room temperature conductivity of the order of 580 Ω–1 cm–1 is obtained, and when the film is doped with indium oxide, an excellent improvement in the conductivity of up to 3.5 × 103 Ω–1 cm–1 is achieved due to the liberation of extra electrons during the process of doping. In all the cases, visible region transparency of above 90 % is attained without significant change due to doping. Structural characterization studies have shown the amorphous nature and smooth surface topography of all undoped and doped films.
References
Aida MS, Tomsella E, Cellier J, Jacquet M, Bouhssira N, Abed S, Mosbah A (2006) Annealing and oxidation mechanism of evaporated zinc thin films from zinc oxide powder. Thin Solid Films 515:1494–1499. doi:10.1016/j.tsf.2006.04.011
Aly SA, El Syed NZ, Kaid MA (2001) Effect of annealing on the optical properties of thermally evaporated ZnO films. Vacuum 61:1–7. doi:10.1016/j.apsusc.2008.11.079
Arredondo EJL, Maldonado A, Asomoza R, Acosta DR, Meléndez-Lira MA, Olvera LM (2005) Indium doped ZnO thin films deposited by sol–gel method. Thin solid films 490:132–136. doi:10.1016/j.tsf.2005.04.043
Banerjee AN, Gosh CK, Chattopadhyay KK, Hideki M, Ajay SK, Atsuya A, Atsushi K, Tamio E (2006) Low temperature deposition of ZnO thin films on PET and glass substrates by DC sputtering technique. Thin solid films 496:112–116. doi:10.1016/j.tsf.2005.08.258
Boris VL, Valer LU, Fedor FG (2004) Kinetics and mechanism of free-surface vaporization of zinc, cadmium and mercury oxides analyzed by the third-law method. Thermochim Acta 411:187–193. doi:10.1016/j.tca.2003.08.024
Bouhssira N, Abed S, Tomsella E, Cellier J, Mosbah A, Aida MS, Jacquet M (2006) Influence of annealing temperature on the properties of ZnO thin films deposited by thermal evaporation. App Surface Sci 252:5594–5597. doi:10.1016/j.apsusc.2005.12.134
Chien YT, Kai-Shiung F, Sih-Han C, Chia-Hao T (2010) Preparation and characterization of ZnO transparent semiconductor films by sol–gel method. J Alloys Compd 495:126–130. doi:10.1016/j.jallcom.2010.01.100
Craciun V, Elders J, Gardeniers JGE, Ian WB (1994) Characteristics of high quality ZnO thin films deposited by pulsed laser deposition. Appl Phys Lett 65: 2963–2965. http://doc.utwente.nl/62540/1/JAPL_65_2963.pdf
Elmer K (2010) Transparent conducting zinc oxide and its derivatives. In: Devid GS, Hideo H, Devid CP (eds) Hand book of transparent conductors, 1st edn. Springer, New York, pp 193–264
Fortunato E, Barquinha P, Pimenentel A, Goncalves A, Marques L, Pereira L, Martins R (2005) Recent advances in ZnO transparent thin films transistors. Thin solid films 487:205–211. doi:10.1016/j.tsf.2005.01.066
Hafdallah A, Yanineb F, Aida MS, Attaf N (2011) In doped ZnO thin films. J Alloys Compd 509:7267–7270. doi:10.1016/j.jallcom.2011.04.058
Jeong SH, Kho S, Jung D, Lee SB, Boo JH (2003) Deposition of aluminum-doped zinc oxide films by RF magnetron sputtering and study of their surface characteristics. Surf Coat Technol 174–175:187–192. doi:10.1016/S0257-8972(03)00600-5
Khairnar UP, Bhavasar DS, Vaidya RU, Bhavasar GP (2003) Optical properties of thermally evaporated cadmium telluride thin films. Mater Chem Phys 80:421–427. doi:10.1016/S0254-0584(02)00336-X
Kykyneshi R, Zeng J, Devid Cann P (2010) Transparent conducting oxide based tin oxide. In: Devid GS, Hideo H, Devid CP (eds) Hand book of transparent conductors, 1st edn. Springer, New York, pp 171–192
Lee C, Jim K, Soong J (1996) Highly textured ZnO thin films doped with indium prepared by pyrosol method. Sol Energy Mater Sol Cells 43:37–45. doi:10.1016/0927-0248(95)00159-X
Lucio LMA, Luna-Arias MA, Maldonado A, de la L Olvera M, Acosta DR (2006) Preparation of conducting and transparent indium-doped ZnO thin films by chemical spray. Sol Energy Mater Sol Cells 90:733–741. doi:10.1016/j.solmat.2006.03.009
Ma QB, Zhi-Zhen Ye, Hai-Ping He, Li-Ping Zhu, Bing-Hui Zhao (2007) Effect of deposition pressure on the properties of ZnO: Ga films prepared by DC magnetron sputtering. Mater Sci Semicond Process 10:167–172. doi:10.1016/j.mssp.2007.11.001
Martinez MA, Herrero J, Gutierrez MT (1997) Deposition of transparent and conductive Al-doped ZnO thin films for photovoltaic solar cells. Sol Energy Mater Sol Cells 45:75–86. doi:10.1016/S0927-0248(96)00066-9
Moon TH, Jeong CM, Woong L, Myoung JM (2005) The fabrication and characterization of ZnO UV detector. App Surf Sci 255:208–211. doi:10.1016/j.apsusc.2004.06.149
Prasad RT, Kumar MCS (2009) Effect of thickness on structural, optical and electrical properties of nanostructured ZnO thin films by spray pyrolysis. App Surf Sci 255:4579–4584. doi:10.1016/j.apsusc.2008.11.079
Prasada RT, Kumar MCS (2010) Physical properties of Ga doped ZnO thin films by spray pyrolysis method. J Alloys Compd 506:788–793. doi:10.1016/j.jallcom.2010.07.071
Rusu GG, Rusu M, Girtan M (2007) Preparation and characterization of ZnO thin films prepared by thermal oxidation of evaporated ZnO thin films. Superlattice Microst 42:116–122. doi:10.1016/j.spmi.2007.04.021
Sahay PP, Nath RK (2008) Al-doped zinc oxide thin films for liquid petroleum gas (LPG) sensors. Sens Actuators B 113:222–227. doi:10.1016/j.snb.2008.02.014
Shigesato Y (2010) In based TCO’s. In: Devid GS, Hideo H, Devid CP (eds) Hand book of transparent conductors, 1st edn. Springer, New York, pp 161–169
Tokumoto MS, Smith A, Santilli CV, Pulcinelli SH, Craievich AF, Elkaim E, Traverse A, Briois V (2002) Structural and electrical properties of undoped and indium doped ZnO thin films prepared by pyrosol process at different temperatures. Thin solid films 416:284–293. doi:10.1016/S0040-6090(02)00531-X
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Palimar, S., Bangera, K.V. & Shivakumar, G.K. Study of the doping of thermally evaporated zinc oxide thin films with indium and indium oxide. Appl Nanosci 3, 549–553 (2013). https://doi.org/10.1007/s13204-012-0161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0161-1