Abstract
ZnO plays an important role in many technological aspects of semiconductors. Because of its interesting properties, it has attracted a great deal of attention for a wide range of applications. In this work, the direct precipitation method was employed for the synthesis of ZnO nanoparticles to study the role of different concentration ratios of reactants on the crystal structure, size, and morphology of the prepared ZnO nanoparticles. The reactant raw materials used in this experiment were zinc acetate dihydrate as a zinc source and NaOH. ZnO nanoparticles were synthesized by calcination of the ZnO precursor precipitates at 250 °C for 3 h. These calcinated ZnO nanoparticles and their properties were characterized using X-ray diffraction, a scanning electron microscope equipped with an energy dispersive X-ray spectrometer, and transmission electron microscopy. We present the experiment conditions, including result on the different reactant concentration ratios, which affect the control of the size and morphology of the ZnO nanoparticles. The mean size of the ZnO nanoparticles was 18 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the more well-known semiconductor materials, ZnO is a II–VI semiconducting ceramic material that possesses a wide band gap of Eg = 3.37 eV and a large exciton binding energy of 60 meV at room temperature (Huang et al. 2001). The importance of this material is reflected in its excellent chemical, mechanical, electrical, optical and optoelectrical properties. For example, it provides both good electrical conductivity (Nakahara et al. 2001) and high transmittance. ZnO has great applicability in the preparation of solar cells (Wang et al. 2001), gas sensors (Lin et al. 1998; Xu et al. 2000), transparent electrodes (Sahu et al. 2007), piezoelectric materials (Wang and Song 2006), and varistors (Koch et al. 1995). However, these are simply a few examples that illustrate its importance. For further indication of its significance, the reader directed to "Appendix".
Nanoparticles are also one of the key elements in nanotechnology. Therefore, in this study we attempt to evaluate the role of different concentration ratios of reactants on ZnO nanoparticles synthesized via the direct precipitation method (DPM). There are also some other parameters that are important in preparation of ZnO nanoparticles by this method and which impact the size and distribution of the nanoparticles, such as the reaction temperature or stirring. Understanding these parameters is important because the properties of these particles depend closely on their size and morphology (Dai et al. 2003).
Over the past twenty years, ZnO nanostructures with various morphologies have been synthesized using a large variety of methods. Such systems provide a rich family of morphologies in comparison to other materials and, as a review of this wide range of nanostructures, we have listed some of these morphologies in "Appendix" so as to present a pertinent background on the works carried out on this important semiconductor material.
Different synthesis methods for the preparation of ZnO nanostructures have been employed in the literature including sol–gel (Tokumoto et al. 2003; Hohenberger et al. 1992), micro-emulsion (Singhal et al. 1997), mechanochemical processing (Tsuzuki and McCormick 2001), vapour phase transport (Zhang et al. 2005), chemical vapour deposition (Wu and Liu 2002), hydrothermal processing (Zhang et al. 2004; Liu and Zeng 2003), spray pyrolysis and drying (Park and Kang 1997; Okuyama and Lenggoro 2003), supercritical-water processing (Viswanthan et al. 2003), thermal decomposition of organic precursor (Rataboul et al. 2002), radio frequency plasma synthesis (Sato et al. 2003), self-assembly (Koh et al. 2004), sonochemical or microwave-assisted synthesis (Hu et al. 2004), homogenous precipitation (Kim et al. 2005) and direct precipitation (Wang and Gao 2003).
In the present study, as mentioned vide supra, ZnO nanoparticles were prepared by precipitation from zinc acetate dihydrate and sodium hydroxide. This method exhibits several advantages over the others listed, such as low cost, large-scale production, low-temperature, and the reduced need for equipment or catalyst assistance. ZnO nanoparticles were obtained and their sizes, morphologies, and structures characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy and transmission electron microscopy (TEM). The ratio of the concentration of zinc acetate dihydrate:sodium hydroxide precursor was varied from 1:1 to 1:4 and the size and morphology of the particles were found to depend strongly on this ratio.
The paper is organized as follows. Previously published works on the synthesis of ZnO nanoparticles were briefly reviewed above. In the section that immediately follows, the main conditions and key parameters of our experiments by the DPM, including the synthesis process, are presented using a schematic diagram. Results obtained by the change in the concentration ratio of the reactant raw materials used in the experiment, and its effect on controlling the size and morphology of the ZnO nanoparticles, are then discussed and show that these properties are size-dependent. Finally, we provide a summary of our findings and present some conclusions.
Material and experimental procedure
Reactant raw materials
Zinc acetate dihydrate (as a Zinc source), sodium hydroxide, ethanol, and distilled deionized water were used in the experiments described herein. Zinc acetate dihydrate and sodium hydroxide were used as the precursors for the formation of ZnO particles.
Synthesis of ZnO nanoparticles
The schematic diagram illustrating the synthesis procedures (DPM) for ZnO particles is presented in Fig. 1.
First, zinc acetate dihydrate (Merck, 99 %) and sodium hydroxide (Merck, 99 %) were each dissolved in distilled deionized water to form liquid media of the desired concentrations. The zinc acetate dihydrate solution was slowly added drop-wise to the sodium hydroxide solution under vigorous stirring at room temperature, forming a transparent white solution. In this way, different concentration ratios of aqueous solutions of zinc acetate dihydrate to sodium hydroxide were prepared. The ratio of the concentrations was varied from 1:1 to 1:4 (zinc acetate dihydrate:sodium hydroxide). These solutions were slowly reacted to produce ZnO particle precipitates. In the next step, the precipitates obtained upon the reaction between the zinc acetate dihydrate and sodium hydroxide solutions were collected by filtration and rinsed three times with distilled deionized water and ethanol. Following this, the washed precipitates were dried in an electric oven at 100 °C for 5 h until ZnO precursors formed. At the final step, the precursors were calcinated at 250 °C for 3 h to obtain ZnO nanopowder.
Results and discussion
X-ray diffraction
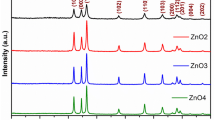
Analysis of the crystal structures of the ZnO nanoparticles prepared by the DPM was performed with an X-ray diffractometer (MMA, GBC, Australia) using Cu Kα radiation. The diffractograms, which are provided in Fig. 2, we recorded in the 2θ range of 20° to 80° with a step size of 0.05° and scan time of 1 s per step.
The four distinct results (a–d) correspond to the different precipitator concentration ratios (zinc acetate dihydrate:sodium hydroxide) of 1:1, 1:2, 1:3, and 1:4, respectively. The prominent peaks labeled on the figure and which were observed at angles of 31.6°, 34.2°, 36.1°, 47.3°, 56.3°, 62.7°, 66.2°, 67.5° and 68.8° belong to the (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes, respectively. These peaks show very good agreement with the reported values of the Joint Committee on Powder Diffraction Standards data (JCPDS 36-1451) and confirm the formation of hexagonal wurtzite zinc oxide (Lepkova et al. 2007; Bhat 2008). No peaks that may correspond to impurities were detected, thus implying that a relatively high purity of ZnO nanoparticles was obtained. EDX spectroscopy was then used to provide elemental analysis and study the compositions of the particles, with the obtained spectrum displayed in Fig. 3.
Figure 3 clearly shows that only Zn and O atoms are present, as evidenced by the determined spectrum. In other words, the product is composed 100 % (Wt and At) of ZnO. We cannot observe the O peaks that must be present from the ZnO because this analysis was carried out using an instrument that cannot depict elements lighter than Na. Figures 2 and 3 both confirm the formation of pure ZnO particles.
The average crystallite size of the obtained particles was measured from the broadening of the diffraction peaks and application of the Scherrer formula (West 1985).
Therein, λ is the wavelength of the Cu kα radiation (1.54 Å), β is the full width at half maximum of the peak, and θ is the Bragg’s angle obtained from the 2θ value corresponding to the maximum intensity peak in XRD pattern. The measured values varied between 20 and 36 nm for samples a–d, as described in Table 1.
As the reactant raw material concentration ratios increases from 1:1 to 1:4, the intensity of the reflections peaks increases, as can be seen from Fig. 2. Therefore, of the samples studied, ZnO nanopowder at a concentration ratio of 1:4 has the most intense peaks and largest crystallite size of 36 nm.
Scanning electron microscopy
The morphologies of the nanoparticles were analyzed using a scanning electron microscope (Philips) equipped with an energy-dispersive X-ray spectrometer. The EDX spectroscopy results were discussed in the previous section.
Figures 4a–d show SEM micrographs of the ZnO particles prepared at different ratios of reactant concentration.
These images clearly show remarkably different morphologies for these ZnO particles. As can be seen from Fig. 4a, at a concentration of 1:1 the nanoparticles are spherical, whereas at a concentration of 1:2, they are cauliflower-like (Fig. 4b). By further increasing the concentration ratio, the clusters become irregularly shaped and non-uniform. As the concentration ratio was gradually increased, the average size of the nanoparticles increased from 18 to 36 nm. From the aforementioned results, it is therefore concluded that the sizes and morphologies of as-prepared ZnO powders depends markedly on the concentration ratio of the reactants. Furthermore, as the nanoparticles become smaller, the surface-to-volume ratio or nanoparticles aspect ratio increases and their chemical reactivity increases.
Transmission electron microscopy
The synthesized ZnO nanoparticles were further characterized using transmission electron microscopy (TEM) (CM10, Philips). Figure 5, which shows a typical TEM image obtained for the sample a, gives further insight into some noteworthy details of the formed particles.
As can be seen from Fig. 5, the ZnO nanoparticles are approximately spherical in shape, confirming the findings obtained by SEM (see Fig. 4a). A histogram (Fig. 6) illustrates the distribution in nanoparticle size; with this figure indicating that a normal distribution of nanoparticles was obtained, centered at a diameter of 15–20 nm. Thus, our samples have a high surface area, making them appropriate for catalytic applications. The higher surface area is attributed to the formation of smaller particles. Photocatalytic activity of ZnO nanoparticles depends on the surface area. Due to the unique properties originating from their high surface area, ZnO nanoparticles and more generally, semiconductor nanoparticles are subjected to much research attentions. Also, synthesis method provides control over the surface area.
Discrepancies in the estimation of nanoparticle size by the different techniques used in this work can be understood. We know that under optimal conditions the precision of the Debye–Scherrer in measuring crystallite size is in the order of ±10 % (Cullity 1978; Azaroff 1968). Therefore, the actual average size of the nanoparticles in this work, as determined by TEM, is 18 nm. However, the nanoparticle size estimated by XRD, which identifies the underlying lattice planes, is 20 nm. Therefore, this value for nanoparticle size is within an acceptable precision limit to the TEM result.
Conclusion
In this work, ZnO nanoparticles were synthesized using the direct precipitation method at room temperature. The advantages of this method are that a large quantity of ZnO nanoparticles can be synthesized with high purity and a low cost of production. The role of different concentration ratios of zinc acetate dihydrate to sodium hydroxide (1:1–1:4) was studied.
X-ray diffraction results show the formation of a hexagonal wurtzite zinc oxide structure with a high degree of crystallinity. By increasing the concentration ratio of the reactant raw materials from 1:1 to 1:4, the intensity of the reflection peaks increased and the average size of the as-prepared nanoparticles increased from 20 to 36 nm.
Scanning electron microscopy images show spherical particles, cauliflower-like particles, and irregular cluster morphologies. It can therefore be concluded that the size and morphologies of as-prepared ZnO powders markedly depends on the concentration ratio.
Transmission electron microscopy revealed the size distribution of the nanoparticles. The actual average size of nanoparticles obtained by TEM for a concentration ratio of 1:1 was 18 nm. The ZnO nanoparticles were approximately spherical, confirming the result obtain by SEM. The nanoparticle sizes estimated given XRD, SEM, and TEM are all in good agreement with each other.
References
Azaroff LV (1968) Elements of X-ray crystallography. McGraw-Hill, USA
Bhat DK (2008) Facile synthesis of ZnO nanorods by microwave irradiation of zinc–hydrazine hydrate complex. Nanoscale Res Lett 3:31
Cullity BD (1978) Elements of X-ray diffraction. Addison Wesley Publishing Company Inc, USA
Dai ZR, Pan ZW, Wang ZL (2003) Novel nanostructures of functional oxides synthesized by thermal evaporation. Adv Funct Mater 13:9
Hohenberger G, Tomandi G, Ebert R, Taube T (1992) Inhomogeneous conductivity in varistor ceramics: methods of investigation. J Am Ceram Soc 74:2067–2072
Hu XL, Zhu YJ, Wang SW (2004) Sonochemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater Chem Phys 88:421–426
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H, Weber E, Russo R, Yang PD (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292:1897
Kim JH, Choi WC, Kim HY, Kang YY, Park K (2005) Preparation of mono-dispersed mixed metal oxide micro hollow spheres by homogeneous precipitation in a micro precipitator. Powder Technol 153:166–175
Koch MH, Timbrell PY, Lamb RN (1995) The influence of film crystallinity on the coupling efficiency of ZnO modulator waveguide. Semicond Sci Technol 10:1523–1527
Koh YW, Lin M, Tan CK, Fao YL, Loh KP (2004) Self-assembly and selected growth of ZnO nanorods on any surfaces supported by an aluminum precoat. J Phys Chem B108:11419–11425
Lepkova K, Clohessy J, Cunnane VJ (2007) The pH-controlled synthesis of a gold nanoparticle/polymer matrix via electrodeposition at a liquid–liquid interface. J Phys Condens Mater 19:375106
Lin HM, Tzeng SJ, Hsiau PJ, Tsai WL (1998) Magnetic and structural properties of nanophase AgxFe1-x solid solution particles. Nanostruct Mater 10:465–477
Liu B, Zeng HC (2003) Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J Am Chem Soc 125:4430–4431
Nakahara K, Takasu H, Fons P, Yamada A, Matsubara K, Hunger R, Niki S (2001) Interactions between gallium and nitrogen dopants in ZnO films grown by radical-source molecular-beam epitaxy. Appl Phys Lett 79:4139–4141
Okuyama K, Lenggoro IW (2003) Preparation of nanoparticles via spray route. Chem Eng Sci 58:537–547
Park SB, Kang YC (1997) Photocatalytic activity of nanometer size ZnO particles prepared by spray pyrolysis. J Aerosol Sci 28:473–474
Rataboul F, Nayral C, Casanove MJ, Maisonnat A, Chaudret B (2002) Synthesis and characterization of monodisperse zinc and zinc oxide nanoparticles from the organometallic precursor [Zn(C6H11)2]. J Organomet Chem 643(644):307–312
Sahu DR, Lin SY, Huang JL (2007) Deposition of Ag-based Al-doped ZnO multilayer coatings for the transparent conductive electrodes by electron beam evaporation. Sol EnergyMater Sol Cells 91:851–855
Sato T, Tanigaki T, Suzuki H, Saito Y, Kido O, Kimara Y, Kaito C (2003) Structure and optical spectrum of ZnO nanoparticles produced in RF plasma. J Cryst Growth 255:313–316
Singhal M, Chhabra V, Kang P, Shah DO (1997) Synthesis of ZnO nanoparticles for varistor application using Zn-substituted aerosol ot microemulsion. Mater Res Bull 32:239–247
Tokumoto MS, Pulcinelli SH, Santilli CV, Briois V (2003) Catalysis and temperature dependence on the formation of ZnO nanoparticles and of zinc acetate derivatives prepared by the sol-gel route. J Phys Chem B 107:568–574
Tsuzuki T, McCormick PG (2001) ZnO nanoparticles synthesized by mechanochemical processing. Scripta Mater 44:1731–1735
Viswanthan R, Lilly GD, Galer WF, Gupta RB (2003) Formation of zinc oxide–titanium dioxide composite nanoparticles in supercritical water. Ind Eng Chem Res 42:5535–5540
Wang JM, Gao L (2003) Synthesis and characterization of ZnO nanoparticles assembled in one-dimensional order. Inorg Chem Commun 6:877–881
Wang ZL, Song JH (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 14:242–246
Wang ZS, Huang CH, Huang YY, Hou YJ, Xie PH, Zhang BW, Cheng HM (2001) A highly efficient solar cell made from a dye-modified ZnO-covered TiO2 nanoporous electrode. Chem Mater 13:678–682
West AR (1985) Solid state chemistry and its application. Wiley, New York, pp 174–175
Wu JJ, Liu SC (2002) Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv Mater 14:215–218
Xu JQ, Pan QY, Shun YA, Tian ZZ (2000) Grain size control and gas sensing properties of ZnO gas sensor. Sens Actuators B Chem 66:277–279
Zhang H, Yang D, Ji Y, Ma XY, Xu J, Que DL (2004) Low temperature synthesis of flowerlike ZnO nanostructures by cetyltrimethylammonium bromide-assisted hydrothermal process. J Phys Chem B 18(13):3955–3958
Zhang Z, Yu H, Shao X, Han M (2005) Near-room-temperature production of diameter-tunable ZnO nanorod arrays through natural oxidation of zinc metal. Chem Eur J 11:3149–3154
Acknowledgment
The authors highly appreciate the support provided by the Karaj Branch of Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
No. | Nano structure | Application | Preparation method | Digital object identifier DOI: |
|---|---|---|---|---|
1 | Nano-arrow | Creating optical or other nanodevices | Atmospheric pressure thermal evaporation | |
2 | Nano-ball | Ultraviolet (UV) lasers | Vapor phase transport | |
3 | Nano-bottles | Creating optical or other nanodevices | Atmospheric pressure thermal evaporation | |
4 | Nano-bowling | Creating optical or other nanodevices | Atmospheric pressure thermal evaporation | |
5 | Nano-belts (Quasi-1D) | Piezoelectric | Chemical vapor phase growth | |
6 | Nano-bridges | Optoelectronics | Thermal evaporation and condensation | |
7 | Nano-cages (1D) | Efficient sensors | Thermolyzing Zn powders | |
8 | Nano-cable (1D) | Functional building blocks in nanoelectronics/optoelectronics | Thermal reduction | |
9 | Nano-candle | Building nanodetectors | Atmosphere pressure physical vapor deposition | |
10 | Nano-cone | Sensors | Atmospheric pressure thermal evaporation | |
11 | Nano-comb | Bio-sensor (glucose detection) | Vapor phase transport | |
12 | Nano-cube | Fabrication of optical and optoelectronic nanodevices | Facile hydrothermal | |
13 | Nano-cluster | Surface acoustic wave (SAW) device fabrication | Matrices of diblock copolymers | |
14 | Nano-double teethed comb | Ultraviolet laser | Chemical vapour transport | |
15 | Nano-disk | Information storage, transducer, light emitter, catalyst and sensor | Vapor-phase transport | |
16 | Nano-ellipsoid like (3D) | Catalysts, sensors and lithium ion electrode materials | Autoclave tests and pyrolysis integrated | |
17 | Nano-fibers | Gas sensor | Electro-spun from solution | |
18 | Nano-flower | Cold cathode electron emission devices | Chemical solution deposition | |
19 | Nano-granular | Gas sensor | Layer-by-layer (LBL) self-assembly | |
20 | Nano-helix(c)es | One dimensional nanoscale sensors, transducers and resonators | Spontaneous polarization-induced of piezoelectric nanobelts | |
21 | Nano-laser | UV nanolaser devices | Vapor phase transport growth | |
22 | Nano-multipod | Ultrafast lasing | Chemical vapor transport and condensation | |
23 | Nano-marigold | Field emission sources | Vapour phase deposition | |
24 | Nano-needle (1D) | Future photoelectric and flat field emission display devices | Electro-chemical deposition | |
25 | Nano-nail | Optoelectronics | Thermal evaporation and condensation | |
26 | Nano-particle | Catalysts for photo-catalytic degradation | Direct precipitation | |
27 | Nano-pillars | Resonator | Chemical vapor deposition | |
28 | Nano-powder | Fabrication of third-generation solar cells | Sol–gel process | |
29 | Nano-prism | UV Lasing | Electrochemical process | |
30 | Nano-pyramid | Field emission sources | Thermal chemical vapor deposition | |
31 | Nano-ribbon (1D) | Laser diodes | Thermal reduction process | |
32 | Nano-ridges | Inverted polymer solar cell | Sol–gel process | |
33 | Nano-ring | Transducers | Physical evaporation of ZnO powder | |
34 | Nano-rod (1D) or 46 | Luminescence, field-emission, gas sensing and electron transport | Vapor phase | |
35 | Nano-shell | Nanocomposite materials | Hydrothermal synthesis | |
36 | Nano-seed | Schottky barrier ultraviolet (UV) photodetectors | Hydrothermal process | |
37 | Nano-spike | Vacuum microelectronic | Vapor–liquid–solid (VLS) | |
38 | Nano-sphere | Gas sensor | Sol–gel technique | |
39 | Nano-spring | Nanoscale electromechanical coupled sensors | Physical evaporation of ZnO powder | |
40 | Nano-sheet (2D) | Gas sensing | Hydrothermal process | |
41 | Nano-spiral | Electromechanical systems | Thermal evaporation | |
42 | Nano-tube (1D) | Dye-sensitized solar cells | Chemical etching | |
43 | Nano-tip | Nanoscale optoelectronic device | Soft chemical | |
44 | Nano-tetrapod | Gas-sensing | Evaporation | |
45 | Nano-wall (2D) | Photocatalysts and opto-electronics | Vapor–liquid–solid (VLS) | |
46 | Nano-wire (1D) or 34 | Optical (high transmittancy in the visible wavelengths) | Homoepitaxial electro-deposition | |
47 | Nano-whisker (1D) or 46 | Micro cavity lasing | Hydrothermal oxidation |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Moghri Moazzen, M.A., Borghei, S.M. & Taleshi, F. Change in the morphology of ZnO nanoparticles upon changing the reactant concentration. Appl Nanosci 3, 295–302 (2013). https://doi.org/10.1007/s13204-012-0147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0147-z