Abstract
Semiconductor nanomaterials tagged with biomarkers may be used for an early fluorescence-based detection of breast cancer. ZnO nanoparticles are water-soluble, non-toxic, photo-chemically stable with highly fluorescence applicability and are regarded for their possible biocompatibility. As a long-term research planning, we are aiming to use QDs conjugated with serum-biomarker for the diagnosis of breast cancer. The present work is a part in the said direction and reports preliminary observations on the synthesis and conjugation of ZnO nanoparticles with a representative protein marker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantum dots (e.g., CdS, CdSe, CdTe, ZnS, PbS) are one of the nanoparticles that are used in imaging, detection and targeting. These are nanometre-size luminescent semiconductor crystals and have unique chemical and physical properties due to their size and their highly compact structure (Nie et al. 2007; Ferrari 2005; Langer 1998; Duncan 2003; Li et al. 2004; Allen 2002). They emit different wavelengths over a broad range of the light spectrum from visible to infrared, depending on their size and chemical composition (Moussodia et al. 2008). Eventual use of nanoparticles to dramatically improve clinical diagnostic tests for the early detection of cancer and other (Meulenkamp 1998; Jain 1998). The use of quantum dots heralds a revolution in biological imaging. The current and widely used organic fluorophores have two shortcomings associated with their fluorescence (Srinivas et al. 2002). Signals from the labelled molecules can be obscured by cell auto fluorescence, occurring in the visible spectrum and by photobleaching, which seriously limits observation time (Neuwalt et al. 2004; Gondal et al. 2009). Colloidal quantum dots are bright, photostable fluorophores of a few nanometers in diameter. Because their size approximates that of individual biomolecule, water-soluble nanoparticles complex have been used to target and image tumour cells. Despite their advantages, the best materials for nanoparticles cadmium sulphide, CdS and cadmium selenide, CdSe can be highly toxic because of quantum confinement region span the entire optical spectrum. Cd ions are able to bind to thiol molecules in mitochondria to cause significant cell death (Gorla et al. 1999). These NPs may also damage DNA and disrupt cell activity from factors such as surface coating them where we prepare biocompatible semiconductor NPs like ZnO remarkable photochemical stability and several properties, including size-dependent broad emission, very high extinction coefficient, readily size-tunable narrow emission, high fluorescence quantum yields and use for disease diagnostics like cancer, neurological disorders etc. (Haase et al. 1988; He et al. 2008; Singh et al. 2005; Thareja and Shukia 2007; Wang et al. 2003; Wong et al. 1998). Proteins conjugation like BSA with nanoparticles attempts have been made for applications such as fluorescence labelling, immunoassays including FRET, disease diagnosis etc. (Mohanta et al. 2008; Xie et al. 2003; Terpetsching et al. 1994). The presence of antibodies that interact with BSA might serve as a diagnostic tool for detection of high-risk patients Kagn Kerman (2007). The presence of antibodies reacting with BSA in sera from HEV-infected patients may play a major pathogenetic role by the generation of autoantibodies.
Presented work includes an overview of applications of fluorescence microscopy for the diagnosis of cancer with special emphasis on ZnO nanoparticles. As an alternative approach for the synthesis of the nanoparticles, chemical route has been suggested for the synthesis of ZnO NPs, which have again been conjugated with BSA. The above steps may be useful for developing an immunosensing method based upon the use of NPs.
Experimental details
Materials and general method
All the solvent and reagents were analytical grade, commercially available and used as received. The UV–VIS spectroscopy (Varian, carry 500) studies were carried out in range 200–3,300 nm. The topological studies were carried out using field emission scanning electrons microscope-energy dispersive X-ray spectroscope (FESEM-EDX Hitachi S4800 SE), dynamic light scattering (DLS) (Brookhaven, BI-200SM). The functionalization and sensing was taken using photoluminescence spectrophometer (Varian, carry 500).
Synthesis of ZnO nanoparticles
The ZnO nanoparticles were synthesized according to Ralph-olivier Moussodia method. Zinc acetate (220 mg) was dissolved in hot ethanol (20 ml) under vigorous stirring. Oleic acid (70 μl) was then added and the mixture was refluxed. In a separate flask, tetramethylammonium hydroxide (360 mg) was dissolved in refluxing ethanol (5 ml). This solution was then rapidly injected in the solution containing zinc acetate and oleic acid. The whole contents were refluxed for 2 min, and then diluted with EtOH (50 ml) followed by cooling to 0 °C. A white precipitate of ZnO nanoparticles appeared. The particles were centrifuged (15 min at 4,000 rpm) for the removal of supernatant. The resulting oleate capped ZnO QDs were washed several times with ethanol. After washing, the ZnO nanoparticles were suspended in 10 ml of toluene and stored in dark at 4 °C.
Conjugation of bovine serum albumin
Before conjugating ZnO nanoparticles with BSA, 5 ml of ZnO suspension was mixed with 5 ml of 5.0 mg/ml NHS (made in 100 μl PBS). After 10 min of reaction, the pH of the solution was adjusted to 7.4 by borate buffer. The conjugation of ZnO nanoparticles with BSA was achieved by incubating the above solution with 1 ml of 1.0 mg/ml BSA for 90 min at 37 °C. The contents were stored at 4 °C.
Results and discussion
Surface morphological studies have been carried out for determining the surface texture of the ZnO nanoparticles. The results of these studies are explained in the following section:
FESEM image of ZnO nanoparticles showing Fig. 1 their morphological parameters and EDX pattern suggests the confirmation for synthesis of ZnO nanoparticles. From both the synthesis routes, the ZnO nanoparticles of <5 nm could be synthesized. However, the agglomeration of these nanoparticles was observed in the case where the procedure 2 was adopted. DLS revealed unimodal population corresponding to dispersion of ZnO nanoparticles in Fig. 2. DLS studies indicates that a Dh of 15–50 nm nanoparticles size range due to some degree of agglomeration.
For long-term storage, it was found more useful to synthesize the ZnO nanoparticles by following the first suggested procedure. UV–Vis spectra of such synthesized nanoparticles are given in Fig. 3. Upon comparison with the reported literature data, it is evident that the prepared nanoparticles have typical characteristics of ZnO crystals and their diameter, calculated by theoretical simulations should lie below 3–4 nm.
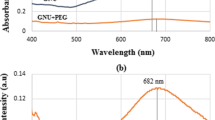
A UV–Vis spectrum of these ZnO nanoparticles, shown in Fig. 2, gives broad absorption band. Such behaviour is typical of ZnO nanoparticles, which are known for broad spectrum UV blocking. Upon conjugation with BSA, new notable absorption peak appears at lower wavelength (218 nm) demonstrating the formation of ZnO-protein conjugate (Fig. 4). Researchers in the past have reported that during nanoparticles-BSA conjugation, the protein undergoes substantial conformational changes at both secondary and tertiary structure levels. These changes are related with the pH of the reaction medium, which determines the extent of blue shift in UV region.
The fluorescence spectra of the ZnO-BSA solutions indicate that the fluorescence spectra changed obviously when ZnO conjugated with BSA. The photoluminescence peak position from 640 nm for pure ZnO emission to 633 nm for ZnO-BSA was accompanied with the decrease in the fluorescence intensity after the addition of BSA (Fig. 5).
Conclusions and future scope
It has been demonstrated that ZnO nanoparticles can be conjugated with BSA. Further studies are required to conclude the mechanism of conjugation, optimization of parameters, fluorescence studies and structural investigations of the prepared conjugate. ZnO nanoparticles or similar kind of fluorescent nanostructures can be conjugated with specific biomarkers for the identification of breast cancer cells.
References
Allen TM (2002) Ligand-targeted therapeutics in anticancer therapy. Nature Rev Drug Discov 2:750–763
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–360
Ferrari M (2005) Cancer nanotechnology: opportunities and challenge. Nat Rev Cancer 5:161–171
Gondal M, Drmosh Q, Yamani Z, Saleh T (2009) Synthesize of ZnO2 nanoparticles by laser ablation in liquid and their annealing transformation into ZnO nanoparticles. Appl Surf Sci 256:298–304
Gorla C, Emanetoglein N, Liang S, Mayo W, Lu Y, Wrabagk M, Shen H (1999) Structural, optical, and surface acoustic wave properties of epitaxial ZnO films grown on (0112) sapphire by metal organic chemical vapor deposition. J Appl Phys 85:2595
Haase AM, Weller H, Henglein A (1988) Photochemistry and radiation chemistry of colloidal semiconductors: 23. Electron storage on zinc oxide particles and size quantization. J Phys Chem 92:482
He C, Sasaki T, Shimizu Y, Koshizaki N (2008) Synthesis of ZnO nanoparticles using nanosecond pulsed laser ablation in aqueous media and the self-assembly towards spindle-like. Appl Surf Sci 254:2196–2202
Jain RK (1998) The next frontier of molecular medicine: delivery of therapeutics. Nat Med 4:655–657
Langer R (1998) Drug delivery and targeting. Nature 392:5–10
Li KCP, Pandit SD, Guccione S, Bednarski MD (2004) Molecular imaging applications in nanomedicine. Biomed Microdevices 6:113–116
Meulenkamp E (1998) Synthesis and growth of ZnO nanoparticles. J Phys Chem B 102(29):5566–5572
Mohanta D, Narayanan SS, Pal SK, Raychaudhuri AK (2008) Fluorescence dynamics of bovine serum albumin (BSA) conjugated CdZnS nanocrystallites. Eur Phys J Appl Phys 43:263–268
Moussodia R (2008) Synthesis and characterization of water-soluble ZnO quantum dots prepared trough peg-siloxane coating. New J Chem 32:1388–1393
Neuwalt EA et al (2004) Imaging of iron oxide nanoparticles with MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol 5:456–471
Nie S, Xing Y, Kim GJ, Simons JW (2007) Nanotechnology applications in cancer. Annu Rev Biomed Eng 9:257–288
Singh J, Srivasatva A, Tiwari R, Srivastava O (2005) Nucleation and growth of catalyst-free zinc oxide nanostructures. J Nanosci Nanotechnol 5:2093–2098
Srinivas PR, Barker P, Srivastava S (2002) Nanotechnology in early detection of cancer. Lab Invest 82:657–662
Terpetsching E, Szmacinski H, Ozinskas A, Lakowicz JR (1994) Synthesis of squaraine-N-hydroxysuccinimide esters and their biological application as long-wavelength fluorescent label. Anal Biochem 217:197–204
Thareja R, Shukia S (2007) Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. Appl Surf Sci 253:8889
Wang Z, Zhang H, Zhang L, Yuan J, Wang C (2003) Low temperature synthesis of ZnO nanoparticles by solid-state. Nanotechnology 11:14
Wong E, Bonevich J, Searson P (1998) Growth kinetics of nanocrystalline ZnO particles from colloidal suspensions. J Phys Chem B 102:777
Xie H, Tkachenko AG, Glomm WR, Ryan JA, Brennaman MK, Papanikolas JM, Franzen S, Feldheim DL (2003) Critical flocculation concentrations, binding isotherms, and ligand exchange properties of peptide-modified gold nanoparticles studied by UV–visible, fluorescence, and time-correlated single photon counting spectroscopies. Anal Chem 75(21):5797–5805
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kumar, P., Kumar, P., Deep, A. et al. Synthesis and conjugation of ZnO nanoparticles with bovine serum albumin for biological applications. Appl Nanosci 3, 141–144 (2013). https://doi.org/10.1007/s13204-012-0101-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0101-0