Abstract
The template-assisted electrodeposition technique has been utilized to synthesize highly ordered, well-aligned, and dense Au–Ni alloy nanowires. The electrochemical impedance spectroscopy has been carried out to study the in situ growth process of alloy nanowires at different electrodeposition times. Their morphological studies have been carried out using the high-resolution transmission electron microscopy. Elemental composition of template-synthesized nanowires has been studied by energy dispersive X-ray analysis. The X-ray diffraction study revealed the face centered cubic structure of the nanowires. Magnetic properties of the nanowires have been studied using Quantum Design vibrating sample magnetometer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanowires are attractive structures for high-performance devices, as their very small sizes, high surface to volume ratios and shape anisotropy impart considerable variation in physical properties that can be exploited for sensor, logic and memory functions, etc. Particularly different research groups show considerable interest in the fabrication and characterization of nanostructured ferromagnetic nanowire arrays due to their potential applications in magnetic recording media and sensors (Fert and Piraux 1999; Albrecht et al. 2000; McGary et al. 2006; Clime et al. 2007), synthesis of carriers for magnetic manipulation in bio-medical applications (Reich et al. 2003; Link and El-Sayed 1999; Nicewarner et al. 2003; Mock et al. 2002) or magnetic nano-assembly (Xu et al. 2005; Bicelli et al. 2008). Because of the possibility of favorable tailoring of their intrinsic and geometric anisotropies (Dahmane et al. 2006; Huang et al. 2002; Shamila et al. 2008; Cho et al. 2006; Kumar et al. 2006), these nanowires are more advantageous over classical superparamagnetic particles. In these days, magnetic multilayered nanowires are potential contenders for high density magnetic data storage, magnetic sensors, and spintronic applications (Fert and Piraux 1999; Albrecht et al. 2000; McGary et al. 2006; Clime et al. 2007). Utilizing the interface phenomena from periodic layering of non-magnetic and magnetic materials with suitable layer thicknesses in multilayered nanowires, giant magnetoresistance (GMR) property can be observed.
Among the various techniques for the growth of nanowires (Kumar et al. 2006; Liu and Bando 2003; Liu et al. 2008; Mehrez and Guo 2004; Monson and Woolley 2003; Toma et al. 2010; Wang et al. 2008; Zhang and Wong 2009; Choi and Park 2004; Zhang et al. 2008, 2000, 2005; Pan et al. 2005), electrodeposition is one of the best and a cost effective method (Martin 1994; Lai and Riley 2008; Mallet et al. 2005; Gupta and Podlaha 2010; Wong et al. 2009). Because using the electrodeposition technique it is very easy to control the thickness of the layers. Because of its selective property, electrodeposition is predominantly suited for making high aspect ratio structures for microelectromechanical systems and other applications. If a template is created from a patterned insulator on a conductive substrate, deposition takes place only where the substrate is uncovered. This is in dissimilarity to evaporation, which coats all over, probably blocking the entrance to particularly fine features in the template. Electrodeposition has an additional advantage, which is the ability to vary composition simply during the growth. If an electrolyte containing ions of metals ‘1’ and ‘2’ is used, and if ‘1’ is more noble than ‘2’, pure ‘1’ will deposit on the cathode at any potential which is adequately negative to reduce ‘1’ but not to reduce ‘2’. When the potential is changed to a value sufficiently negative to reduce ‘2’, both metals will be deposited, but assuming the concentration of ‘1’ in the electrolyte is sufficiently low, the resulting alloy will predominately be ‘2’ (Kazeminezhad and Schwarzacher 2008).
The understanding of electrodeposition mechanism is necessary for controlling and tuning the properties of electrodeposited nanowires. Electrochemical impedance spectroscopy (EIS) is the best tool for achieving this purpose (Riveros et al. 2008). This technique is simple and nondestructive. In the present work, in situ EIS was used to examine the interface of porous electrode and electrolyte. Its behavior during the growth of Au–Ni nanowires was also studied. The structural and magnetic properties of Au–Ni nanowires were also reported.
Experimental
The commercially available anodic alumina membrane (AAM) purchased from Whatman, UK, having average pore diameter 100 nm and pore density 109 cm−2, has been used for the fabrication of Au–Ni alloy nanowires. The chemicals and reagents used were of analytical grade (Fisher Scientific, purity 99.5 %). The de-ionized water was used for preparing solutions. The electrodeposition process has been carried out in a typical three-electrode electrochemical cell using Potentiostat (Gamry Reference 600) with a platinum electrode as counter electrode, Ag/AgCl2 as reference electrode, and the AAM coated with silver paste as working electrode. The electrolytic bath contains HAuCl4·3H2O (10 mM), NiCl2·6H2O (0.5 M), and H3BO3·2H2O (0.5 M) whereas the current density was controlled via a Gamry Reference 600 potentiostat/galvanostat. For the generation of alloy nanowires, pulsed electrodeposition technique was used, by periodically switching the potential between −200 mV (30 s) for Au and −800 mV (10 s) for Ni. Figure 1a presents the applied pulsed potential during the growth of Au–Ni nanowires and the resulting current response is shown in Fig. 1b.
For studying the in situ growth of Au–Ni alloy nanowires, EIS experiments were carried out at different electrodeposition times. Initially, we applied the pulsed potentials for 200 s, afterward the electrochemical growth was stopped and then the in situ EIS was performed at a low overpotential of −15 mV with 5-mV ac perturbation (Riveros et al. 2008). The frequency range used was 1 MHz–0.2 Hz. After that, the growth process was restarted and the similar procedure was repeated for different deposition times.
The crystallographic study of Au–Ni nanowires embedded in AAM as host was performed using the X-ray diffractometer (PANalytical X’Pert) with CuKα (k = 1.5406 Å) radiation operated at 45 kV and 40 mA. For transmission electron microscopy (TEM) characterization, the prepared sample of Au–Ni nanowires embedded in the AAM was put in a solution of NaOH to dissolve the alumina membrane. The nanowires were then washed several times with de-ionized water followed by ethanol. A small drop of the above solution, containing the nanowires, was taken on carbon-coated copper grids for the TEM characterizations. Energy dispersive X-ray (EDX) analyzer attached with high-resolution transmission electron microscopy (HR-TEM) was used to confirm that nanowires prepared using electrodeposition had the desired composition throughout their length. Quantum design VSM was used to study the magnetic properties of Au–Ni nanowires.
Results and discussion
Figure 2a represents the impedance diagrams in the Nyquist plane for Au–Ni nanowire arrays within the AAM at different growth times. As the electrodeposition time increases the resistance decreases, predicting an important contribution of the internal pore resistance to the total resistance, which diminishes as the pore is being filled. The possible equivalent circuit for the metal/AAM electrode is shown in Fig. 2b. Cc corresponds to the electrical capacitance of the AAM, Re the ohmic drop across the electrolyte, Rp the electrical resistance of the pores, Rct is attributed to the charge transfer resistance, W represents the Warburg impedance characterizing the diffusion process and Cdl corresponds to the double layer capacitance (Sima et al. 2008).
Figure 3a presents the SEM micrograph of Au–Ni nanowires embedded in AAM. It clearly depicts the large scale and uniform growth of nanowires. Some hemispherical caps on the top of AAM show the over-deposition. Figure 3b depicts the HR-TEM micrograph of a nanowire with backscattered mode and selected area electron diffraction (SAED) pattern. SAED shows the crystalline nature of Au–Ni nanowires. The surface of the nanowires observed was not smooth. It got ruptured due to the dissolution of AAM in NaOH solution with high concentration (2 M). Figure 3c shows the HR-TEM of nanowires with EDX at different positions. EDX analysis of nanowires confirmed the presence of more than 93 % of gold in the nanowires. The large time period (30 s) for gold deposition than nickel (10 s) is the reason for high percentage of gold in the template-synthesized nanowires. Gold also co-deposited with nickel at 800 mV required for nickel deposition.
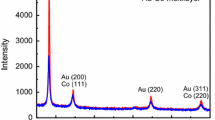
The X-ray diffraction (XRD) analysis of the Au–Ni nanowires, embedded in AAM, has been carried out to study crystal structure (Fig. 4). It shows the presence of the four reflection peaks attributed to planes, (111), (200), (220), (311), confirming gold nanowires with the face centered cubic crystal structure (JCPDS 04-0784). From Fig. 4, it is also clear that the growth of Au–Ni nanowires is preferably along the direction (111). One reflection peak for nickel (111) is also observed overlapping with gold peak (200), but with low intensity may be due to low percentage of nickel.
The hysteresis curves for arrays of Au–Ni-alloyed nanowires are shown in Fig. 5. Inset of Fig. 5 shows clear ferromagnetic signal in Au–Ni-alloyed nanowires. The hysteresis loops were measured using a magnetic field applied perpendicularly and parallel to the axes of the nanowires at room temperature (300 K). The magnetic behavior of Au–Ni-alloyed nanowires is strongly dependent on the effective magnetic anisotropy and dipole–dipole interaction among the wires. Magnetic anisotropy further depends upon shape, magneto-crystalline and magneto-elastic contributions (Cortex et al. 2000). In the case of long nanowires, the easy axis is along the nanowire axis, as the preferred magnetization direction is dominated by the nanowire shape anisotropy. In our case ferromagnetic signal is very small due to the small concentration of Ni in the nanowires, as observed by EDX analysis. Ferromagnetic signal is more enhanced in the case of field applied parallel to the wire axes as compared to the field applied perpendicular to the wire axes. Coercivity observed was also more in the case of field applied parallel to the wire axes. The high pore density in the case of alumina template, consequences in high interwire interactions which stimulate a dipolar field due to neighboring wire interactions. This dipolar field will act as a demagnetization field. The effect of high dipolar interactions for perpendicular fields results in the reduced magnetization (Narayanan et al. 2008).
Conclusions
Au–Ni nanowires were successfully synthesized using electrodeposition in the pores of AAM. SEM confirms the uniform growth of nanowires. EDX analysis confirmed that the Ni concentration is very small as compared to gold because of small time period for Ni deposition and high concentration of gold. Using EIS it was observed that with increase in electrodeposition time, pore resistance decreases. From TEM micrographs, we found that high concentration of NaOH for the dissolution of host AAM is harmful for the template-synthesized nanowires. Small ferromagnetic signal is observed in Au–Ni alloy nanowires may be due to small concentration of Ni as compared to Au. High dipolar interactions among nanowires for perpendicular fields result in poor magnetization.
References
Albrecht TT, Schotter J, Kästle GA, Emley N, Schibauchi T, Elbaum LK, Guarini K, Black CT, Tuominen MT, Russell TP (2000) Ultrahigh-density nanowire arrays grown in self-assembled diblock copolymer templates. Science 290:2126
Bicelli LP, Bozzini B, Mele C, Durzo L (2008) A review of nanostructural aspects of metal electrodeposition. Int J Electrochem Sci 3:356
Cho JU, Wu JH, Min JH, Ko SP, Soh JY, Liu QX, Kim YK (2006) Control of magnetic anisotropy of Co nanowires. J Magn Magn Mater 303:e281
Choi H, Park SH (2004) Seedless growth of free-standing copper nanowires by chemical vapor deposition. J Am Chem Soc 126:6248
Clime L, Zhao SY, Chen P, Normandin F, Roberge H, Veres T (2007) The interaction field in arrays of ferromagnetic barcode nanowires. Nanotechnology 18:435709
Cortex A, Riveros G, Palma JL, Denardin JC, Marotti RE, Dalchiele EA, Gomez H (2000) Single crystal growth of nickel nanowires: influence of deposition conditions on structural and magnetic properties. J Nanosci Nanotech 9:1992
Dahmane Y, Cagnon L, Voiron J, Pairis S, Bacia M, Ortega L, Benbrahim N, Kadri A (2006) Magnetic and structural properties of electrodeposited CoPt and FePt nanowires in nanoporous alumina templates. J Phys D Appl Phys 39:4523
Fert A, Piraux L (1999) Magnetic nanowires. J Magn Magn Mater 200:338
Gupta M, Podlaha EJ (2010) Electrodeposition of CuNiW alloys: thin films, nanostructured multilayers and nanowires. J Appl Electrochem 40:1429
Huang YH, Okumura H, Hadjipanayis HC (2002) CoPt and FePt nanowires by electrodeposition. J Appl Phys 91:6869
Kazeminezhad I, Schwarzacher W (2008) Electrodeposited Ni–Cu alloy nanowires with arbitrary composition. Electrochem Solid State Lett 11:K24
Kumar A, Fähler S, Schlörb H, Leistner K, Schultz L (2006) Competition between shape anisotropy and magnetoelastic anisotropy in Ni nanowires electrodeposited within alumina templates. Phys Rev B 73:064421
Lai M, Riley DJ (2008) Templated electrosynthesis of nanomaterials and porous structures. J Coll Int Sci 323:203
Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103:8410
Liu Z, Bando Y (2003) A novel method for preparing copper nanorods and nanowires. Adv Mater 15:303
Liu N, Wu D, Wu H, Liu C, Luo F (2008) A versatile and “green” electrochemical method for synthesis of copper and other transition metal oxide and hydroxide nanostructures. Mater Chem Phys 107:511
Mallet J, Zhang KY, Tempfli SM, Tempfli MM, Piraux L (2005) Electrodeposited L10 Co x Pt1-x nanowires. J Phys D Appl Phys 38:909
Martin CR (1994) Nanomaterials: A membrane-based synthetic approach. Science 266:1961
McGary PD, Tan L, Zou J, Stadlera BJH, Downey PR, Flatau AB (2006) Magnetic nanowires for acoustic sensors. J Appl Phys 99: 08B310
Mehrez H, Guo H (2004) In: Wang Z (ed) Nanowires and nanotubes materials, properties and devices-metal and semiconductor nanowires, vol 1. Springer, Berlin
Mock JJ, Oldenburg SJ, Smith DR, Schultz DA, Schultz S (2002) Composite plasmon resonant nanowires. Nano Lett 2:465
Monson CF, Woolley AT (2003) DNA-templated construction of copper nanowires. Nano Lett 3:359
Narayanan TN, Shaijumon MM, Ci L, Ajayan PM, Anantharaman MR (2008) On the growth mechanism of nickel and cobalt nanowires and comparison of their magnetic properties. Nano Res 1:465
Nicewarner-Peña SR, Carado AJ, Shale KE, Keatin CD (2003) Barcoded metal nanowires: optical reflectivity and patterned fluorescence. J Phys Chem B 107:7360
Pan H, Liu B, Yi J, Poh C, Lim S, Ding J, Feng Y, Huan CHA, Lin J (2005) Growth of single-crystalline Ni and Co nanowires via electrochemical deposition and their magnetic properties. J Phys Chem B 109:3094
Reich DH, Tanase M, Hultgren A, Bauer LA, Chen CS, Meyer GJ (2003) Biological applications of multifunctional magnetic nanowires. J Appl Phys 93:7275
Riveros G, Gomez H, Schrebler R, Marotti RE, Dalchiele EA (2008) An in situ EIS study during the electrochemical growth of copper nanowires into porous polycarbonate membranes. Electrochem Solid State Lett 11:K19
Shamila S, Sharif R, Riaz S, Ma M, Rahman MK, Han XF (2008) Magnetic and magnetization properties of electrodeposited fcc CoPt nanowire arrays. J Magn Magn Mater 320:1803
Sima M, Enculescu I, Sima M, Vasile E, Visan T (2008) EIS studies if electrodeposition process of manganese and copper doped ZnO wires. Surf Interface Anal 40:561
Toma A, Chiappe D, Boragno C, Mangeot FB (2010) Self-organized ion-beam synthesis of nanowires with broadband plasmonic functionality. Phys Rev B 81:165436
Wang C, Hu YJ, Lieber CM, Sun SH (2008) Facile synthesis of ultrathin and single-crystalline Au nanowires. J Am Chem Soc 130:8902
Wong J, Greene P, Dumas RK, Liu K (2009) Probing magnetic configurations in Co/Cu multilayered nanowires. Appl Phys Lett 94:032504
Xu CL, Zhang L, Zhang HL, Li HL (2005) Well-dispersed gold nanowires suspension for assembly application. Appl Surf Sci 252:1182
Zach MP, Ng KH, Penner RM (2000) Molybdenum nanowires by electrodeposition. Science 290:2120
Zhang F, Wong SS (2009) Controlled synthesis of semiconducting metal sulfide nanowires. Chem Mater 21:4541
Zhang J, Sun J, Wei W, Shi S, Sun H, Guo J (2005) Synthesis of copper nanowires under a direct current electric field. Nanotechnology 16:2030
Zhang DW, Chen CH, Zhang J, Ren F (2008) Fabrication of nanosized metallic copper by electrochemical milling process. J Mater Sci 43:1492
Acknowledgments
This work is supported by Department of Science and Technology (DST) under Nano Mission scheme (Sanction Order No. SR/NM/NS-84/2008). The authors are grateful to DST for its generous funding. Authors are also thankful to Prof. M.K. Wu, IOP, Academia Sinica, Taipei for providing HRTEM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kumar, S., Saini, D. Large-scale synthesis of Au–Ni alloy nanowires using electrochemical deposition. Appl Nanosci 3, 101–107 (2013). https://doi.org/10.1007/s13204-012-0098-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0098-4