Abstract
The IFT and contact angle are believed to have direct impact on wettability alteration of crude oil/water/rock systems. In this work, extensive laboratory work was conducted to investigate the effect of these two key parameters on wettability alteration at elevated temperature (90 °C) and ambient conditions. Twenty-six brines representing various scenarios of dilution and sulphate spiking were prepared and tested to identify the smart water most effective in the alteration of wettability. Sea water (SW) was used a base brine. Diluted and sulphate-spiked versions of SW were synthetically prepared following the standard brine preparation procedures. Also standard procedures were followed for the measurement of IFT and contact angle measurements using Teclis tracker. Pendant drop method was implemented to measure the IFT at ambient and 90 °C conditions using a special software package that adopts the axisymmetric drop shape analysis and fits the Laplace equation. The same software package was used to take snapshots of oil drops at 90 °C, and contact angle was measured manually. The effects of dilution and/or sulphate spiking on the observed IFT and contact angle measurements were investigated using a proposed brine categories-based plotting technique. Natural SW and its sulphate-spiked versions have shown the least oil/brine IFT at ambient and 90 °C conditions. The sulphate-spiked SW and its dilutions have resulted in the reduction in oil/brine IFT, whereas the diluted SW showed an increase in oil/brine IFT. Further reduction in IFT was observed at the elevated temperature. SW and SW/50 (50 times diluted sea water) were the only two brines that could yield a contact angle of 113° and 114°, respectively, indicating the change in wettability from oil-wet to the border line of intermediate-wettability conditions. The natural SW that contains 3944 mg/L of sulphate ion has been found to be the most effective in promoting wettability change and thus represents the selected smart water for EOR implementation in Asab oil field. The contact angle measurements were made from the drops formed by the natural drainage process. These measurements are believed to duplicate contact angles in the selected reservoir because of the continuous change in fluids saturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Half of the world’s hydrocarbon reserves is occupied by carbonate rocks. The mechanism that governs the oil recovery should be known for a successful oil production. An important factor that controls the fluid distribution in a reservoir is formation wettability. Most carbonate reservoirs are preferentially oil-wet and exhibit negative capillary pressure. These reservoirs exhibit reduced oil recovery compared to sandstones because of their fractured nature. The permeability of the matrix block is in the range of 1–10 mD which makes carbonate reservoirs good candidates for enhanced oil recovery. Most of the petrophysical parameters like capillary pressure, relative permeability, electrical properties and waterflood behaviour are dependent on wettability (Alotaibi et al. 2010; Hognesen et al. 2005). Consequently, any wettability alteration would affect the above parameters and eventually the whole flooding process.
If the wettability is between water-wet and intermediate-wet, injected water will be spontaneously imbibed by the matrix block (Torsaeter 1984). In an oil-wet rock, negative capillary pressure will make the spontaneous imbibition impossible, whereas in a fractured oil-wet reservoir, the injected water moves through the high permeable fractures and results in early water breakthrough (Al-Hadhrami and Blunt 2000).
Wettability alteration studies between sea water and rock gained momentum after the successful injection of sea water into the highly fractured Ekofisk field in the North Sea (Torsaeter 1984; Zhang et al. 2007). Calcium and sulphate have been found to exhibit strong potential towards the calcite surfaces (Pierre et al. 1990). Also low-salinity flooding has proven to be effective in some carbonate reservoirs (Al-Attar et al. 2013; Zahid et al. 2012). No extensive work was done to find the effect on increased sulphate ion concentration in sea water on possible wettability change. In this work, extensive laboratory effort was made on the measurement of key properties at 90 °C and ambient temperature which are believed to have direct impact on wettability alteration of crude oil/water/rock systems. The results of contact angle and IFT measurements of different brines were analysed to have a better understanding of the effect of dilution, sulphate spiking and temperature in wettability alteration.
Wettability
Wettability is defined as the relative adhesion of two fluids to a solid surface. In a porous medium, it is a measure of preferential tendency of one of the fluids to wet the surface. A porous medium usually contains two or more fluids (Tiab and Donaldson 2010).
Depending on the brine–oil–rock interaction, the wettability of a system ranges from strongly water-wet to strongly oil-wet. Brine–oil–rock system will exhibit neutral wettability, if rock does not show any preference to either brines. Or in other words, neutral wettability is defined as a condition when both fluids equally wet the rock surface (Tiab and Donaldson 2010).
Fractional wettability is a type of wettability where scattered areas of the rock are strongly oil-wet and the remaining area is strongly water-wet. Fractional wettability is also known as “Dalmatian wetting” (Brown and Fatt 1956; Willhite 1986). It occurs when the surface of the rock is composed of many minerals having different surface chemical properties, which leads to a change in wettability throughout the internal surface of the pores. The core exhibiting fractional wettability will imbibe small amount of oil when water saturation is high like at residual oil saturation (S or) and will imbibe a small quantity of water when oil saturation is high like at irreducible water saturation (S wi).
Mixed wettability is defined as condition where larger pores are oil-wet and a continuous filament of oil exists throughout the core in larger pores, whereas the small pores are occupied by water (Anderson 1986; Salathiel 1973; Willhite 1986). Residual oil saturation of mixed wettability is low because oil is located in the large pores of the rock in continuous path that makes the oil displaced from the cores even at very low oil saturation. Mixed wettability can occur when oil containing interfacial active polar organic compounds invades a water-wet rock saturated with brine. After displacing brine from the larger pores, the interfacial active compounds react with the rock’s surface, displacing the remaining aqueous film and, thus, producing an oil-wet lining in the large pores. The water film between the rock and the oil in the pore is stabilized by a double layer of electrostatic forces. As the thickness of the film is diminished by the invading oil, the electrostatic force balance is destroyed and the film ruptures, allowing the polar organic compounds to displace the remaining water and react directly with the rock surface.
So the overall average characteristic of a heterogeneous system with microscopic relative wetting throughout the porous medium is the wettability of a rock–fluid system (Iwankow 1958). The preferential wetting tendencies of water or oil towards the rock pore surfaces lead to various states of overall wettability. This overall wettability has an effect on the fluid flow and electrical properties of the water–hydrocarbon–rock system. It is capable of controlling the capillary pressure and relative permeability behaviour that leads to the hydrocarbon displacement and ultimate recovery (Donaldson and Thomas 1971; Emery et al. 1970; Kyte et al. 1961; Masalmeh 2002).
Wettability alteration mechanism
In carbonate reservoirs, wettability alteration is the main challenge in displacing more oil and enhancing the oil recovery (Alotaibi et al. 2010). Strand et al. (2008) investigated the effect of calcium, magnesium and sulphate ions on oil recovery. For any wettability improvement, activation energy for the chemical reaction is required. Bonding energy between the polar components in oil and carbonates is high compared to sandstones. Also the carbonate rock is capable of absorbing the carboxylic component in the crude oil onto carbonate surface, and because of this, wettability always remains between neutral and preferential oil-wet. Sulphate ion is capable of acting as a wettability modifier without any addition of surfactants. Sulphate is an ion that showed up good potential towards the limestone (Pierre et al. 1990; Strand et al. 2003, 2008).
In an imbibition test using seawater, the effect of ions (sulphate and calcium) with temperatures seems to have a crucial role in wettability alteration. An increase in the concentration of calcium in sea water increases the adsorption of sulphate; this is because of the co-adsorption of calcium ion towards the carbonate surface. The positive charge of the rock surface decreases with adsorption of sulphate onto the carbonate rocks; because of reduced electrostatic repulsion, it increases the calcium ions at the surface (Austad et al. 2007; Strand et al. 2006, 2008). Adsorption of sulphate onto chalk surface leads to the desorption of negatively charged carboxylic material by changing the surface charge of the chalk surface (Strand et al. 2003). Temperature increase leads to a strong adsorption of sulphate and calcium onto the chalk surface, which enhances the imbibition rate and oil recovery. At low temperature, adsorption of magnesium ions is less compared to calcium ions onto the chalk surface (Zhang and Austad 2006; Zhang et al. 2007). The increase in temperature replaces calcium on the chalk surface by magnesium. Magnesium becomes more reactive because of dehydration and gets replaced instead of calcium from the surface lattice of the chalk. The presence of sulphate, calcium and magnesium is necessary to change the wettability of rock. Limestone also showed similar interactions with sea water (Alotaibi et al. 2010).
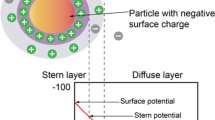
The wettability of carbonate rocks was studied by Lichaa et al. (1992) for preserved and cleaned core samples. Rock/fluid interaction can be evaluated by contact angle, Amott and USBM. In a brine/crude oil/rock system, the surface charges on the rock surface and fluid interfaces are strongly affected by the salinity and pH of the brine, which in turn affects the wettability. The presence of cations like calcium, magnesium and strontium in the formation water of injection water and the weak base characteristic of reservoir rock suggest a preferential oil-wet system should prevail in the presence of polar components in the crude oil. pH of the brine has an effect on the wetting nature, when the zeta potential crosses the zero point of charge.
Interfacial tension
When two immiscible fluids (gas–liquid or liquid–liquid) are in contact, the fluids are separated by a well-defined interface, which is of only a few molecular diameters in thickness. Within the fluid and away from the interface and the walls of the container, the molecules attract each other in all directions. At the surface between two immiscible fluids, there are no similar molecules beyond the interface and, therefore, there is an inward-directed force that attempts to minimize the surface by pulling it into the shape of a sphere. This surface activity creates a film-like layer of molecules that are in tension, which is a function of the specific free energy of the interface. The interfacial tension (IFT) has the dimensions of force per unit length (newton/metre), which is the modern standard expression of the units. In the earlier literature, however, it is expressed as dynes/centimetre, which is numerically equal to millinewtons per metre [(N × 10−3)/m or mN/m] (Tiab and Donaldson 2010).
During the development phase and to implement an optimal reservoir management strategy for a reservoir, the knowledge about the reservoir fluid properties is very important (Amyx et al. 1988). IFT and contact angle are important parameters for any reservoir engineering studies. They can be used in the estimation of fluid saturation in gas–oil transition zone (Tiab and Donaldson 2010). No general analytical method is available for estimating IFT, so it has to be measured in the laboratory for reservoir samples at reservoir conditions (Okasha and Al-Shiwaish 2010).
The study of oil/brine IFT is closely related to wettability. So IFT and film formation can help to explain the change in contact angle and wettability. Pressure was found to have less effect on IFT compared to temperature. So temperature is considered as a major factor affecting IFT (Hjelmeland and Larrondo 1986).
Contact angle
Contact angle is a function between solid/liquid and liquid/liquid interfaces. Wettability of the reservoir rocks shows a thermodynamical equilibrium between the mineral surface of the pore walls and fluid within the pores. Wettability is a function of pressure, temperature, fluid characteristics and reservoir heterogeneity. Contact angle is affected by the heterogeneity and roughness of solid wall and affects the hysteresis. The contact angle hysteresis is the difference between advancing (maximal) contact angle and receding (minimal) contact angle where advancing contact angle to receding contact angle is a range of contact angles when a drop is placed on the surface of rock. Contact angle of 0° and 180° means completely water-wet and completely oil-wet, respectively. Anderson (1986) classified the wettability in terms of contact angle as water-wet (0°–75°), intermediate-wet (75°–115°) and oil-wet (115°–180°). Weakly water-wet and weakly oil-wet conditions are represented as (55°–75°) and (115°–135°), respectively.
Hjelmeland and Larrondo (1986) studied the effect of temperature, pressure and oil composition on the wettability of the calcium carbonate rocks. They concluded that the temperature had an influence on the wettability. At low temperature (72 °F), the rock surface was oil-wet, and at high temperature (>140 °F), rock surface showed water-wet behaviour. An intermediate state of wettability was observed at 104 °F. There was no effect of pressure on wettability. Light fraction of oil had no effect on the wettability of calcium carbonate.
Saner et al. (1991) studied a carbonate reservoir using contact angle, Amott and USBM. Synthetic brines with salinity ranging from 20 to 200,000 ppm were used with crude oil under elevated temperature and pressure conditions. He concluded that an increase in temperature from ambient to 158 °F would change the wettability from neutral wet to moderately water-wet conditions. Also an increase in salinity from 20 to 200,000 ppm was found to decrease the contact angle from 61° to 42°. Low-salinity brines did not show any significant change in contact angle between ambient and elevated temperature (158 °F) conditions. Pressure was found to have no influence on the contact angle, as the pressure was increased from 20 to 2800 psia at constant temperature (158 °F). Salinity effect was almost negligible at similar temperature conditions.
Lichaa et al. (1992) studied the wettability of Saudi Arabian carbonate reservoirs using the contact angle, Amott and USBM technique. The receding contact angle measurement of the calcite, marble and formation rock was made using the synthetic formation water, sea water and dead oil. The experiment was conducted at different pressures (ambient to 50 psia) and different temperatures (77–194 °F). They found that at high temperature, calcite surface became preferential water-wet. The contact angle of brine/marble/oil shows oil-wet to intermediate-wet, and at high temperatures wettability changed to weakly water-wet. Formation rocks showed oil-wet at room temperature and weakly oil at high temperature.
The effect of pressure and temperature on reservoir rock wettability was investigated by Wang and Gupta (1995). Stock tank oil and reservoir brine from a carbonate reservoir was used. Pressure had no major effect on the contact angle of the calcite rock; an increase in contact angle was only 5% when there was an increase of 3000 psig pressure. An increase in temperature from 72.5 to 175 °F changed the wettability of the system towards weakly water-wet. A change in the fluid chemistry at the interface with an increase in temperature leads to the change in wettability.
Almehaideb et al. (2004) investigated the effect of salinity on the carbonate rock. Limestone rock, crude oil and NaCl solution were used in the study. Distilled water, 1000, 10,000 and 50,000 ppm of brines were used. All the experiments were conducted at room temperature. 10,000 ppm brine showed a significant reduction in contact angle compared to other brines.
Yu et al. (2007) studied the effect of the brine containing sulphate on the chalk rock. They measured the contact angle on calcite and chalk rocks at high temperatures (up to 266 °F). A temperature of 194 °F helped to change the wettability of calcite towards water-wet. Accelerated desorption of the stearic acid from the calcite helped to change the wettability of the all fluid systems investigated towards water-wet. Replacing distilled water by sulphate-containing water resulted in a decrease in contact angle. Also a decrease in contact angle was observed when sulphate-containing water was used at high temperatures around 266 °F.
The wettability of the crude oil/reservoir brine/reservoir rock system was evaluated at elevated temperatures using axisymmetric drop shape analysis (ADSA) technique by Yang et al. (2008). Vuggy limestone of intermediate wettability was used in the study. An increase in contact angle was observed with an increase in pressure. At 29 psia pressure and 80.6 °F temperature, a slight fluctuation of contact angle was observed. This slight fluctuation might be because of the strong electrostatic interaction between crude oil and reservoir brine. A decrease in contact angle was observed with an increase in temperature.
The advancing and receding contact angles were measured as a function of temperature by Hamouda and Karoussi (2008). All the contact angle measurements were made on modified calcite surfaces with 0.005 M stearic acid dissolved in decane. A maximum temperature of 194 °F was used in the experiments. An increase in temperature reduced the contact angle, indicating system is becoming more water-wet with the temperature increase. This happens because of the total interaction potential, which consists of van der Waals attractive, short-range Born repulsive and double-layer electrostatic forces.
Methodology
Asab oil field
The crude oil and core samples were taken from the Asab onshore oil field in UAE, operated by Abu Dhabi Company for Onshore Petroleum Operation Ltd (ADCO). The field was discovered in 1965 and is located approximately 185 km South of Abu Dhabi, in rolling sand dunes some 30 km north of the Liwa oasis. The reservoir rock is carbonates with total proven reserves of 3.6 billion barrels of oil, and current production rate is about 450,000 barrels per day. The current average reservoir pressure is around 3100 psia with a temperature of 255 °F.
Crude oil
Reservoir crude oil from the Asab field was used in all experiments. The dead oil density and viscosity at 20 °C are 0.8276 g/cc and 2.93 cp, respectively. The oil is sweet and has no H2S gas. The oil was filtered through a 5-µm filter paper in the presence of vacuum to remove any solid particles.
Brines
In this study, a total of 26 brines were used including formation water (FW) and injection water (IW) of Asab field. All brines were prepared using Schlumberger manuals. From the literature, sea water has shown good recovery in carbonate reservoirs (RezaeiDoust et al. 2009; Zhang et al. 2007). Also the effect of sulphate ions in water has shown some additional oil recovery. Sea water was collected from the Arabian Gulf, the water body close to that of Asab field, and its ionic analysis was performed. Sea water of Total Dissolved Solids (TDS) 57,539 mg/L was selected as base brine and was synthetically prepared in the laboratory. Different brines were prepared by diluting the sea water and by spiking the sea water with sulphate. Spiking was based on the multiples of the original 885 mg/L of sulphate presents in formation water. Brines were spiked by 1770 mg/L (×2 SO4) and 5310 mg/L (×6 SO4). Na2SO4 salt was used for sulphate spiking, and ionic balance calculation was performed to assure the sulphate spiking is done properly. As stated in the literature review, the ×3 SO4 and ×4 SO4 spiking have been found to increase oil recovery (Zhang and Austad 2005). Therefore, a sulphate spiking of ×6 SO4 was attempted in this work to see how it could alter the IFT and contact angle measurements. Formation water and injection water samples were collected from the field and subjected to ionic analysis. Asab oil field has a formation water of TDS 157,488 mg/L with a density of 1.1034 g/ml and viscosity of 1.3483 cp at ambient conditions. The injection water of the field has a TDS of 258,250 mg/L with a density of 1.1639 mg/L and viscosity of 1.75 cp at ambient conditions.
Brine composition
Table 1 shows the composition of all brines used in the work. Ionic analysis was performed to find the composition of formation water, injection water and sea water. The brine composition of sea water dilutions and sulphate spiking was thus calculated and cross-checked by ionic analysis.
Core samples
Four core samples were selected from well number 567 in Asab field. The properties of the core samples are listed in Table 2 indicating all the core samples are limestone. Also all core samples are horizontal sections, mentioned as “H” in the column of sample number. Each core sample was cut into 3 pieces horizontally because trim ends are required for contact angle measurements and named as sample nos. 1, 2 and 3. A core sample is shown in Fig. 1. A piece of trim end was obtained by cutting the shortened core sample and used for contact angle measurements.
Core preparation
Standard Core Lab procedures were implemented in cutting, trimming and cleaning the core samples. Core samples are provided by ADCO and are cylindrical in shape, 4″ in length and 1.5″ in diameter. The core samples were cut into three horizontal pieces using the core trimming machine. For cleaning, Soxhlet extraction apparatus was used. The core samples were placed in medium of toluene and then in the medium of methanol. Toluene was used to extract hydrocarbon and methanol to remove salts. Then, all cleaned core samples were placed in oven for drying.
Density and viscosity measurements
Density measurements of all brine were conducted by pycnometer. Cannon–Fenske viscometer was used to measure the dynamic viscosity.
Interfacial tension measurements
All Interfacial Tension (IFT) measurements of oil/brine were carried out using Teclis tracker as shown in Fig. 2 by pendant drop technique. It is a technique by which a drop of liquid is suspended from the end of a tube by surface tension. Teclis tracker is capable of running IFT measurements at ambient and high-temperature conditions, and IFT was recorded at both conditions. The upper limit of temperature with Teclis tracker is 90 °C.
Contact angle measurement
All contact angle measurements were performed on rock samples aged by fully saturating in Asab crude oil for three weeks at 90 °C, making the rock surface oil-wet with deduced contact angle of 180°. Contact angle measurements were made at 90 °C and 248 psia. The oil-wet rock samples placed in a medium of brine were subjected to the above conditions. Teclis tracker took pictures of the naturally popped up drop above the surface of the rock at regular intervals for 72 h, starting from nearly oil-wet condition. Teclis tracker can focus only on a single drop at a time.
Results and discussion
IFT results of different brines at 20 °C
IFT of all the brines were measured at 20 °C and ambient pressure. All runs were carried out at a constant volume until stabilized IFT was obtained. The stabilized value of the interfacial tension in dyne/cm at the end of each IFT test has been recorded and tabulated as presented in Table 3. Figure 3 is prepared on the basis of data from Table 3 and the categories listed in Table 4. A trendline was drawn for each category to generalize the behaviour of brines in that category.
Category 1 shows a decreasing trend of IFT, similar to that observed by Okasha and Alshiwaish (2009). These authors studied the effect of salinity on IFT and concluded that the decrease in salt concentration from 200,000 mg/L to 50,000 mg/L did reduce the IFT. They named the 50,000 mg/L brine as low-salinity brine. The reduction in IFT results in the weakening of the intermolecular forces between oil and brine which assisted by the gravity effects promotes oil detachment from the brine. Category 1 also includes three different natural brines (SW, FW and IW) without dilution or sulphate spiking. The SW shows the least IFT compared to FW and IW, which is due to least amount of TDS in the SW.
Category 2 shows an increase in IFT with the effect of sulphate spiking. The IFT of six times sulphate-spiked brine of SW is 5.41% greater than that of SW with natural sulphate. This increase in IFT in category 2 is due to the increased amount of sulphate by 5310 mg/L in the spiked brine.
Categories 3–9 show the combined effect of dilution and sulphate spiking. Categories 3–7 show a declining trend of IFT with an increased concentration of sulphate. In category 3, the IFT of six times sulphate-spiked brine of SW/10 is 11% less than that of SW/10 without sulphate spiking. In category 4, the IFT of six times sulphate-spiked brine of SW/50 is 7.8% less than that of SW/50 without sulphate spiking. In category 5, the IFT of six times sulphate-spiked brine of SW/100 is 7.8% less than that of SW/100 without sulphate spiking. In category 6, the IFT of six times sulphate-spiked brine of SW/200 is 4.7% less than that of SW/200 without sulphate spiking. In category 7, the IFT of six times sulphate-spiked brine of SW/300 is 11.6% less than that of SW/300 without sulphate spiking. This reduction in IFT in categories 3–7 is due to the increased amount of sulphate by 5310 mg/L in the spiked brine. So in categories 3–7, effect of sulphate spiking is more dominant than the effect of dilution. Observational error in categories 8 and 9 lead to an increasing trend of IFT. The same is to be confirmed from the IFT at high-pressure and high-temperature conditions because the sulphate has more effect at higher temperatures. In category 8, the IFT of six times sulphate-spiked brine of SW/400 is 6% greater than that of SW/400 without sulphate spiking. In category 9, the IFT of six times sulphate-spiked brine of SW/500 is 19.8% greater than that of SW/500 without sulphate spiking. This increase in IFT in categories 8 and 9 is due to the increased amount of sulphate by 5310 mg/L in the spiked brine. So effect of dilution is more than the effect of sulphate spiking in categories 8 and 9.
Category 10 shows the effect of dilution. Categories 11 and 12 show the combined effect of dilution and spiking. The categories 10–12 have large number of brines compared to the brines in the categories 2–9. An increase in IFT was observed for categories 10–12, because of the reduction in ions with dilutions. Six times sulphate-spiked brine of SW/500 has more amount of sulphate ion compared to other ions in the same brine, but still no promising IFT was observed.
The SW (categories 1, 2 and 10), SW ×2 SO4 (categories 2 and 11) and SW ×6 SO4 (categories 2 and 12) are the three brines that show the least IFT in Table 3 with SW ×2 SO4 showing the least IFT. Any further dilution from SW and the sulphate spiking of diluted SW would not be sufficient to reduce the IFT.
IFT of brines at 90 °C
SW, SW/10, SW/50 and their respective sulphate-spiked brines that showed comparatively less IFT at 20 °C were selected as candidates for IFT measurement at 90 °C. Six more brines with relatively higher IFT at 20 °C were also selected for the same purpose, to have an idea how the high-temperature condition can affect the IFT measurements of these two sets of brines. Also IFT of formation water and injection water were measured at 90 °C. All IFT measurements were obtained at 90 °C and 248 psi. Pressure has been found to have a little effect on IFT (Hjelmeland and Larrondo 1986). In this work, pressure was applied to prevent evaporation of brine at the elevated temperature. Table 5 lists the observed IFT values of different brines at 90 °C. All runs were continued until a stabilized IFT was obtained. Brines of lesser IFT with Asab crude oil were considered for further contact angle measurements. The reduced IFT promotes oil detachment from the brine surface, and more oil will be recovered. Figure 4 is prepared on the basis of data from Table 5 and categories defined in Table 6. A trendline was drawn for each category to generalize the behaviour of brines in that category. The discussion that follows is based on Fig. 4. Lesser IFT will promote oil detachment, and in that context brines are considered to show a favourable effect.
High-temperature condition is found to reduce the IFT values of category IFT 1 significantly compared to IFT at 20 °C. Among the three brines in this category, SW corresponds to the least value of TDS and results in the least IFT. The formation and injection water samples, however, show high values of IFT even at 90 °C. There is an increasing trend in IFT for the category IFT 1.
Wang and Gupta (1995) concluded that the increase or decrease in IFT values depends on the composition of the brine. From categories IFT 2–5, there is a decreasing trend of IFT. Category 2 shows the effect of sulphate spiking. Combined effect of dilution and sulphate spiking is observed in categories 3–5. The three brines in categories 2–5 mainly differ in the concentration of sulphate ion, and an overall reduction in IFT with sulphate spiking at 90 °C can be observed. The increased concentration of sulphate in the brine at 90 °C is found to reduce the IFT. In category IFT 2, as the sulphate concentration is increased from 3944 to 9254 mg/L, the IFT decreased by 12.2%. The IFT of SW decreased by 29.5% at 90 °C compared to 20 °C and ambient pressure conditions. In category IFT 3, as the sulphate concentration is increased from 394 to 5704 mg/L, the IFT decreased by 11.83%. In category IFT 4, as the sulphate concentration is increased from 79 to 5389 mg/L, the IFT decreased by 6.26%. In category IFT 5, as the sulphate concentration is increased from 10 to 5320 mg/L, the IFT decreased by 15%.
An increasing trend of IFT in categories 6, 7 and 8 illustrates the effect of brine dilution on IFT measurements at 90 °C. In category IFT 6, there is 94.8% increase in IFT between SW/400 and SW, which is quite significant. During dilution of brines, the concentration of potential ions like calcium, magnesium and sulphate was reduced, which lead to an increase in IFT with dilution. In category IFT 7, there is 60% increase in IFT by going from SW ×2 SO4 to SW/400 ×2 SO4. Even though all the brines were twice spiked and had more sulphate compared to category IFT 6, IFT was slightly reduced. In category IFT 8, there is 88.5% increase in IFT by going from SW ×6 SO4 to SW/400 ×6 SO4 with. During the dilution of six times sulphate brines, concentration of potential ions like calcium, magnesium and sulphate was reduced; diluted brines had higher sulphate compared to other ions in the brine. These higher sulphate ions, however, were not able to reduce the IFT.
The SW, SW ×2 SO4 and SW ×6 SO4 are the three brines that showed the least IFT in Table 5 with SW ×6 SO4 showing the least IFT of these three brines. Any further dilution of SW and the sulphate spiking of diluted SW would not be sufficient to reduce the IFT. Thus, the observed IFT trends at 90 °C are in good agreement with those at 20 °C.
IFT measurements with temperature
The IFT was measured with temperature to see the effect of temperature on IFT. Nine best brines that showed the least IFT at 90 °C were selected as the candidates for IFT measurement with temperature. IFT values were recorded with temperature varying from 20 to 90 °C and pressure varying from 200 psi to 248 psi. The system was pressurized to avoid evaporation of the brine. The IFT values versus temperature are listed in Table 7.
In Fig. 5, the IFT of all brines shows a declining trend with temperature. The percentage reduction in IFT between 20 and 90 °C is shown in Fig. 6. So the temperature plays a key role in lowering the IFT. From Fig. 6, the highest percentage reduction in IFT is for SW/10 ×2 SO4 (−46.55%) and the lowest percentage reduction in IFT is for SW ×2 SO4 (−19.56%). Flock et al. (1986) and Karnanda et al. (2012) observed similar trend in IFT with increased temperature.
Contact angle measurements at 90 °C
Temperature, pressure and fluid characteristics are strongly believed to have an effect on wettability (Alotaibi et al. 2010). In this work, all contact angle measurements were performed on aged rock samples in oil, making the rock surface oil-wet. A zero contact angle represents the condition of a fully water-wet system. Neutral wettability is considered at 90 °C. The brines that exhibited least IFT were selected as candidate for contact angle. All contact angle measurements were carried out at 90 °C and 248 psi. The aim is to verify how far the brine is capable of changing the wettability. Alotaibi et al. (2010) and Anderson (1986) classified wettability in terms of contact angle as being water-wet (0°–75°), intermediate-wet (75°–115°) and oil-wet (115°–180°). Weakly water-wet and oil-wet conditions are represented by (55°–75°) and (115°–135°), respectively. Some contact angle measurements were also carried out for brines of high IFT values at 90 °C. Stabilized contact angle measurements after elapsed time of 72 h are given in Table 8. Figure 7 is based on data from Table 8 and categories from Table 9. All the discussions are based on Fig. 4 and trendline of each category in Fig. 7. Figure 8 shows the change in contact angle at 90 °C and 248 psi condition (Table 6).
In category CA 1, sulphate spiking of the SW seems to change the rock surface to become more oil-wet. The natural SW (Δθ = 67°) was capable of changing the wettability from oil-wet to the border line of intermediate-wet system. In category CA 2, SW/10 (Δθ = 49°) changed the wettability from oil-wet to weakly oil-wet. In category CA 3, SW/50 (Δθ = 66°) changed the wettability from oil-wet to border line of intermediate wettability. Hognesen et al. (2005) reported that the ratio of calcium to sulphate ion is a key factor in altering the wettability. It seems like sulphate spiking was not enough to achieve that optimum calcium/sulphate ratio. Although this observation contradicts the results of Pierre et al. (1990), Strand et al. (2003, 2008) concluded that sulphate is the ion that shows good potential towards limestone. Contact angle is dependent on temperature and independent of pressure (Wang and Gupta 1995). All measurements were made at 90 °C to incorporate that effect.
Categories CA 4 and 5 show an increase in contact angle with dilution. It seems that calcium/sulphate ratio was not good enough to alter the wettability. In category CA 6, the contact angle was decreased but did not change the wettability from oil-wet to intermediate-wet.
SW was thus selected as the most likely smart brine from the observations of this work because it had the least contact angle and changed the wettability from oil-wet to the border line of intermediate-wet conditions. Also the IFT of SW is among the least. Also the SW/50 changed the wettability from oil-wet to border line of intermediate-wet conditions, but it does not cater for dilution cost with deionized water. It seems like sea water having the optimum ratio of sulphate to calcium ions because sea water changed the wettability from oil-wet to the border line of intermediate-wet. Ratio of sulphate and calcium ions of SW, SW ×2 SO4 and SW ×6 SO4 is 5.72, 8.28 and 13.41, respectively. So, a ratio of 5.72 may be considered as the optimum ratio of sulphate to calcium in this work. All the six times sulphate-spiked brine stood strongly in oil-wet nature.
SW and SW/50 are the brines that changed the wettability from oil-wet to the border line of intermediate-wet conditions at 90 °C and 248 psi condition.
Conclusions
-
1.
Based on the results of IFT measurements at 20 °C, SW and its twice and six times sulphate-spiked may be considered as the three best brines that have shown the least IFT. Among these three brines, the SW ×2 SO4 brine has shown the lowest IFT.
-
2.
The results of IFT measurements at 90 °C have shown that SW, its twice and six times sulphate-spiked seem to be the three best brines of least IFT. Increasing the test temperature from ambient condition to 90 °C has been found to reduce the IFT. Among these three brines, the SW ×6 SO4 brine has shown the lowest IFT, because the sulphate ion was capable of interacting more at 90 °C.
-
3.
From the contact angle results at 90 °C and 248 psi, the best brines that showed the least contact angle are SW and SW/50. These brines changed the wettability of rock from oil-wet to border line of intermediate-wet.
-
4.
Sulphate spiking at 90 °C has shown further reduction but higher contact angles.
-
5.
Brine dilution at 90 °C has been found to increase both IFT and contact angle.
-
6.
From economic point of view, SW is the most likely smart water which has an IFT of 9.503 dyne/cm and a contact angle of 113 degrees (Δθ = 67°), at 90 °C and 248 psi.
Abbreviations
- CA:
-
Contact angle
- Cat.:
-
Category
- FW:
-
Formation water
- IFT:
-
Interfacial tension
- IW:
-
Injection water
- SW:
-
Sea water
- SW/y :
-
Sea water/y times diluted
- SW/y ×Z SO4:
-
Sea water/y times diluted and that spiked by Z times the sulphate of formation water (885 mg/L)
- TDS:
-
Total dissolved solids
References
Al-Attar HH, Mahmoud MY, Zekri AY, Almehaideb RA, Ghannam MT (2013) Low salinity flooding in a selected carbonate reservoir: experimental approach. Soc Pet Eng. doi:10.2118/164788-MS
Al-Hadhrami HS, Blunt MJ (2000) Thermally induced wettability alteration to improve oil recovery in fractured reservoirs. Soc Pet Eng. doi:10.2118/59289-MS
Almehaideb RA, Ghannam MT, Zekri AY (2004) Experimental investigation of contact angles under oil-microbial solution on carbonate rocks. Pet Sci Technol 22(3–4):423–438. doi:10.1081/LFT-120024568
Alotaibi MB, Azmy R, Nasr-El-Din HA (2010) Wettability challenges in carbonate reservoirs. Soc Pet Eng. doi:10.2118/129972-MS
Amyx JW, Bass DM, Whiting RL (1988) Petroleum reservoir engineering: physical properties. McGraw-Hill, New York
Anderson WG (1986) Wettability literature survey—part 1: rock/oil/brine interactions and the effects of core handling on wettability. J Pet Technol 38(10):1125–1144. doi:10.2118/13932-PA
Austad T, Strand S, Madland MV, Puntervold T, Korsnes RI (2007) Seawater in chalk: an EOR and compaction fluid. Int Pet Technol Conf. doi:10.2523/11370-MS
Brown RJS, Fatt I (1956) Measurements of fractional wettability of oil fields’ rocks by the nuclear magnetic relaxation method. Soc Pet Eng. doi:10.2118/743-G
Donaldson EC, Thomas RD (1971) Microscopic observations of oil displacement in water-wet and oil-wet systems. Soc Pet Eng. doi:10.2118/3555-MS
Emery LW, Mungan N, Nicholson RW (1970) Caustic slug injection in the singleton field. J Petrol Technol 22(12):1569–1576. doi:10.2118/2425-PA
Flock DL, Le TH, Gibeau JP (1986) The effect of temperature on the interfacial tension of heavy crude oils using the pendent drop apparatus. J Can Pet Technol. doi:10.2118/86-02-06
Hamouda AA, Karoussi O (2008) Effect of temperature, wettability and relative permeability on oil recovery from oil-wet chalk. Energies 1(1):19–34. doi:10.3390/en1010019
Hjelmeland OS, Larrondo LE (1986) Experimental investigation of the effects of temperature, pressure, and crude oil composition on interfacial properties. SPE Reserv Eng 1(04):321–328. doi:10.2118/12124-PA
Hognesen EJ, Strand S, Austad T (2005) Waterflooding of preferential oil-wet carbonates: oil recovery related to reservoir temperature and brine composition. Soc Pet Eng. doi:10.2118/94166-MS
Iwankow EN (1958) A correlation of interstitial water saturation and heterogeneous wettability. Dissertation: Master of Petroleum Engineering University of Oklahoma, 1958
Karnanda W, Benzagouta MS, AlQuraishi A, Amro MM (2012) Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab J Geosci 6(9):3535–3544. doi:10.1007/s12517-012-0605-7
Kyte JR, Naumann VO, Mattax CC (1961) Effect of reservoir environment on water-oil displacements. J Pet Technol 13(06):579–582. doi:10.2118/55-PA
Lichaa PM, Alpustun H, Abdul JH, Nofal WA, Fuseni AB (1992) Wettability evaluation of a carbonate reservoir rock. In: Worthington PF, Chardaire-Riviera C (eds) Advances in core evaluation III-reservoir management, Gordon and breach science publisher, Great Britain, 409–412
Masalmeh SK (2002) Studying the effect of wettability heterogeneity on the capillary pressure curves using the centrifuge technique. J Pet Sci Eng 33(1–3):29–38. doi:10.1016/S0920-4105(01)00173-5
Okasha TM, Alshiwaish A (2009) Effect of brine salinity on interfacial tension in Arab-D carbonate reservoir. Soc Pet Eng, Saudi Arabia. doi:10.2118/119600-MS
Okasha TM, Al-Shiwaish A-JA (2010) Effect of temperature and pressure on interfacial tension and contact angle of Khuff Gas reservoir, Saudi Arabia. Soc Pet Eng. doi:10.2118/136934-MS
Pierre A, Lamarche JM, Mercier R, Foissy A, Persello J (1990) Calcium as potential determining ion in aqueous calcite suspensions. J Dispers Sci Technol 11(6):611–635. doi:10.1080/01932699008943286
RezaeiDoust A, Puntervold T, Strand S, Austad T (2009) Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels 23(9):4479–4485. doi:10.1021/ef900185q
Salathiel RA (1973) Oil recovery by surface film drainage in mixed-wettability rocks. J Pet Technol 25(10):1216–1224. doi:10.2118/4104-PA
Saner S, Asar HK, Okaygun H, Abdul HJ (1991) Wettability study of Saudi Arabian carbonate reservoir core samples. Arab J Sci Eng 16(3):357–371
Strand S, Standnes DC, Austad T (2003) Spontaneous imbibition of aqueous surfactant solutions into neutral to oil-wet carbonate cores: effects of brine salinity and composition. Energy Fuels 17(5):1133–1144. doi:10.1021/ef030051s
Strand S, Høgnesen EJ, Austad T (2006) Wettability alteration of carbonates—effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf A 275(1–3):1–10. doi:10.1016/j.colsurfa.2005.10.061
Strand S, Puntervold T, Austad T (2008) Effect of temperature on enhanced oil recovery from mixed-wet chalk cores by spontaneous imbibition and forced displacement using seawater. Energy Fuels 22(5):3222–3225. doi:10.1021/ef800244v
Tiab D, Donaldson EC (2010) Petrophysics: theory and practice of measuring reservoir rock and fluid transport properties (2. ed. with new and updated materials, [reprint.]). Elsevier, Amsterdam
Torsaeter O (1984) An experimental study of water imbibition in chalk from the Ekofisk field. Soc Pet Eng. doi:10.2118/12688-MS
Wang W, Gupta A (1995) Investigation of the effect of temperature and pressure on wettability using modified pendant drop method. Soc Pet Eng. doi:10.2118/30544-MS
Willhite GP (1986) Waterflooding (3. printing). Society of Petroleum Engineers, Richardson
Yang D, Gu Y, Tontiwachwuthikul P (2008) Wettability determination of the crude oil–reservoir brine–reservoir rock system with dissolution of CO2 at high pressures and elevated temperatures. Energy Fuels 22(4):2362–2371. doi:10.1021/ef800012w
Yu L, Standnes DC, Skjæveland SM (2007) Wettability alteration of chalk by sulphate containing water, monitored by contact angle measurement. Presented at the international symposium of the society of core analysts, Calgary, Canada
Zahid A, Shapiro AA, Skauge A (2012) Experimental studies of low salinity water flooding carbonate: a new promising approach. Soc Pet Eng. doi:10.2118/155625-MS
Zhang P, Austad T (2005) The relative effects of acid number and temperature on chalk wettability. Soc Pet Eng. doi:10.2118/92999-MS
Zhang P, Austad T (2006) Wettability and oil recovery from carbonates: effects of temperature and potential determining ions. Colloids Surf A 279(1–3):179–187. doi:10.1016/j.colsurfa.2006.01.009
Zhang P, Tweheyo MT, Austad T (2007) Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf A 301(1–3):199–208. doi:10.1016/j.colsurfa.2006.12.058
Acknowledgements
Financial support and samples of this work are gratefully acknowledged from the Abu Dhabi Company for Onshore Petroleum Operations Ltd. (ADCO). Also authors would like to thank Petroleum Institute, Abu Dhabi, for their support and cooperation in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abubacker, J., Al-Attar, H., Zekri, A. et al. Selecting a potential smart water for EOR implementation in Asab oil field. J Petrol Explor Prod Technol 7, 1133–1147 (2017). https://doi.org/10.1007/s13202-017-0315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-017-0315-5