Abstract
The best understanding of the geochemical process of groundwater is important for a sustainable development and for a strategic management of water resources. A hydro-geochemical study of groundwater of the Djelfa multilayer aquifers systems was established, using stable isotopes, and major elements, in order to identify the groundwater mineralization process occurring in this system. The chemical data indicate that the dissolution of evaporate minerals (Gypsum, halite, anhydrite) and the evaporation from water table are the principle processes who command the groundwater mineralization. Groundwater samples are depleted in stables isotopes (18O and 2H) reflecting a slug of water recharged during colder climatic conditions, and the recharge was occurred at different altitude. The analysis of the replenishment of aquifer levels shows that the level arises in the replenishment of aquifer and decreases in the depletion of aquifer due to the phenomena of evaporation and to the extensive pumping from wells. Excessive pumping creates a remarkable cone of depression that makes flow direction from syncline to the basin outlet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The groundwater in the region of Djelfa is the main source to supply the population and the agricultural and industrial activities. In this research, we have used the hydrochemistry and the isotopic characteristics of the groundwater as tools to assess the hydrogeological system and the geochemical interactions between the water and rocks to identify the origin of these waters. The region was characterized by semiarid climate, with irregular hydro-climatic conditions, due to high perturbation observed in the atmospheric circulation. In the Hydrogeological scale, the region was formed by multilayer’s aquifer, as first we find the aquifer of the mio-plio-quaternary deposits, the Turonian aquifer, the Barremian aquifer and the Albian aquifer. Water Hydrochemistry is variable at the spatial scale, generally is controlled by the interaction with the bedrock, the contact with some saline rocks determine groundwater mineralization. Mineralization is low in the south of the region and high in the north, which expresses a high degree of heterogeneous bedrock and geological complicated factors. In general, we see that the waters of the region are old; it is clear in their poorly content of heavy isotope. The combinations between isotopic and hydro-chemical characteristics of waters let us to appreciate the geochemical interaction and recharge processes.
We aim in this paper to study the geochemical process and mineralization of groundwater in the Syncline of Djelfa. By introducing the geochemical and isotopic tools, the results obtained showed a different type of water that belongs to three aquifers systems, and the isotopic analysis shows that these samples are poor in heavy isotopes which mean that the waters of these aquifers are old. The recharge process was downed in another cold period which is different from the actual climatic conditions.
Materials and methods
Study area
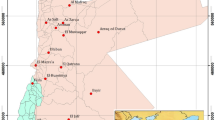
The study site is located in the region of Djelfa (Fig. 1), the syncline of Djelfa is composed from multilayer aquifers system with a variable depth, and the productivity of the aquifers depends on the geologic and hydraulic parameters of each layer.
Climatic aspect
Djelfa is under Mediterranean climate (semiarid type), with a cold and rainy winter and a hot, dry summer (Fig. 2), with annual rainfall of around 336 mm. The inter-annual average temperature is 14.6 °C. The calculation of the water balance by the method of Thornthwaite revealed that evapotranspiration gives a value of 804.66 mm and an ETR of 278.12 mm (Chibane 2010).
Geologic settings
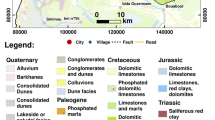
The study area comprises different geological deposits (Fig.3). The Barremian deposits consist primarily of alternating sandstones and sandy clays that are intensely cracked, form a productive aquifer 1,500 to 2,000 m, thick. The lower Albian consists primarily of continental sandstones with dense cracking and there are multiple springs. Its thickness is about 400 meters and is one of the most important aquifers. The Turonian is primarily limestone with marls alternations near the top. The density of these limestone fractures indicates that the aquifer is karstified. Its thickness is 450 meters. The Mio-plio-Quaternary mainly sandy, Silty and conglomeratic is surmounted by a calcareous crust. Due to its low permeability, the Mio-plio-Quaternary is mined by small wells, and its thickness is variable (250–300 m) (Chibane and Ali Rhmani 2015).
Hydrogeology setting
The region of Djelfa was characterized by the presence of Multilayer aquifer system, which has a variable potentiality in point of view water resources. The density of fractures of the cretaceous deposit promotes a significant flow and recharge process in winter time. Three potential layers are used to supply the city of Djelfa. Theses layers are formed by the Turonian, the Barremian and the Albian aquifers.
From the hydrogeological point of view, the sandstone of the Barremian intensely fractured; bordering the plain is an important productive aquifer.
In addition to the Albian which formed mainly of continental cracked sandstones. It is the place of the emergence of several sources and also forms an important aquifer. Turonian limestone aquifer is a very productive (Chibane et al. 2015).
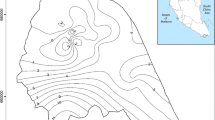
Water table maps (Fig. 4a and b) show the flow direction and piezometric line in two different period (September, 2001 and May 2002). The principle direction of flow is (West East and South North) following the direction of river flow.
Sampling strategy
Eighteen samples were collected from boreholes and spring sources, for geochemical and isotopic analysis. Water samples, for ions concentration analysis, were filtered and acidified (1% v/v HNO3), and collected in 500 mL polyethylene bottles. Samples were filtered through 0.45 mm membranes to eliminate the solids particles. Samples for stable isotopes analysis are collected in 60 ml rinsed and distilled bottles.
Samples analysis
Field parameters are determined by a portable multiparameters device (PCE-PHD instrument) where the pH, the CE, the Temperature, the TDS, and the Eh are determined, whereas potassium (K +), calcium (Ca 2 +), sodium (Na +), magnesium (Mg 2 +), nitrate (NO − 3), sulfate (SO−24), and chloride (Cl −) contents were determined by ion chromatography. Isotopic analyzes were carried out by using a spectrometer of mass laser-based technology of type (PICARO) in the Nuclear research center of Algiers (CRNA).
Results and discussion
The in situ parameters (Temperatures, Electrical conductivity, and Total dissolved salts) are reported in Table 1.
The groundwater temperature is a good indicator of the origin of water which show the depth where these waters come from, the contact with high depth and big geologic fault. The in-site measurement show that the temperature ranges between 16 °C measured in well (OS.8) and 40 ◦C in spring of Hamam Charef (Sp.01).
High temperature value shows that water of Hamam Charef has a high depth origin, this temperature signified the presence of deep fault. The pH of the analyzed waters samples is in a narrow range (7.35–8.53); the pH values show neutral water to near basic.
The electric conductivity measured in groundwater samples extends from 518 µS/cm to 2030µS/cm with an average of 1071.61 µS/cm. High electric conductivity indicates high dissolved minerals in these waters, which express the high exchange between the geological layers and water. The corresponding mineralization expressed as total dissolved salt (TDS) contents varies from 341 mg/l measured in the well (OS2) and 1400 mg/L measured in the well (T). High TDS values characterize waters extremely rich in mineral salts indicating evaporites deposits leaching.
The major ions concentration results are reported in Table 2. Statistical summary is given in Table 3
The statistical summary shows that the concentrations of major element are variable from well to other; this variability can be explained by the geologic nature of the host rock its type and its mineralogical composition.
The mean concentration of the Calcium (Ca + +) is around 118 mg/l with a low concentration about 20 mg/l measured in the spring source (Rocher de sel). The high concentration was measured in the well (T). The calcium origin is the dissolution of the carbonate rock (CaCO3) and the gypsum rocks. The magnesium concentration depends on the nature of land entered in contact with infiltrated waters.
Secondly, the magnesium concentration has high amplitude, with an average of 33.2 mg/l where the minimum value has measured in the well (OS1) with a concentration of 2.37 mg/l. The maximum value has measured in the well (OS12) with a concentration about 75.89 mg/l.
Thirdly, the sodium also varies between 3.90 mg/l measured in the well (OS8), and the highest concentration of sodium was measured in the well of (Hamam Echaref) with 114 mg/l; also, the potassium concentrations significantly are low, with an average of 8.17 mg/l; the low value was measured in the spring source (Rocher de sel) with 2.90 mg/l, and the maximum value was measured in the well (DF4) with 13.10 mg/l.
The bicarbonate concentration is average of 284.67 mg/l, the minimum was measured in the well (OS4), and the maximum value was measured in the well (T) with a value of 414.80 mg/l.
Finally, the concentration of sulfate ion is variable in all the well and spring sources (Fig. 5), the average value is about 167.57 mg/l with a minimum value observed in the well (OS2) with 66.20 mg/l, (Fig. 5) the maximum value is measured in the Turonian well (T) with 378.80 mg/l, and the chloride concentration has different sources and origin (Atmospheric deposit, dissolution of salt); in our region the Triassic age has a high influence on the mineralization of the groundwater as we see in the Turonian aquifer (presence of slug of salt and gypsum in alternance).
The average concentration of chloride is about 89.38 mg/l, and the minimum value was measured in the well (OS2) with 41.13 mg/l. The higher value is around 239.66 mg/l, and it is observed in (OS12). As a conservative element, the major source of chloride is the rainfall and atmospheric deposits in the regions; as we know, the saline lack in the north of region may form an another source of groundwater chloride.
Statistical summary of geochemical analysis of groundwater samples, show a high fluctuation of NO3—,Na+,Cl−, Mg++,SO4−−,Ca++,K+ and HCO3−, variation coefficients are respectively 185, 85, 64, 64, 59, 52, 38 and 24%.
The variation coefficient correspond to the nitrate composite (NO3-), is very high which express a media with high risk and vulnerability to a diffuse pollution result from the agricultural activities, and urban water rejects. These variations signify the high heterogeneous of groundwater in the regions due to the geological nature of the aquifer.
The ionic ratio in Table 4 shows clearly the dominance of geochemical reaction; direct exchange or reversed ionic exchange.
The sample of OSF1, OSF10, OSF2, OSF5 and OSF7, has a domainance of carobante rock, the Rocher de sel sample has a dominance of sodium exchange which mean a probably contact with salty rocks.
Figure 6 shows the 2d diagram of Na in function of chloride concentration, and all samples follow sea water dissolution, except the sample N° 17.
The N°17 sample (Rocher de sel) has a contact with evaporate rock riches in sodium composition. (water type: Na-HCO3).
Where Na/Cl molar ratio is approximately one, halite dissolution is responsible for the sodium, whereas a ratio greater than one is typically interpreted as Na released from a silicate weathering reaction (Meybeck, 1987). In this study, the molar ratio of Na/Cl for groundwater samples of the study area generally ranged from 0.08 to 1,27. Majority of the samples have molar ratio less 1 indicating that cation exchange is the major process, and the sample Socher de seld has a ration above one indicate ion exchange which is replaced by silicate weathering (Srinivasamoorthy et al. 2011). The excess of Na can be attributed to silicate weathering (Stallard and Edmond, 1983) from feldspars.
Isotopic analysis
The primary results of stable isotopic analysis obtained in this work are shown in Tables (5 and 6), and the graphic plots in Fig. 7 show the isotopic content in groundwater samples of the Djelfa Syncline.
As we see, the result shows poor water in heavy isotopes and the variation of the deuterium isotope is between (− 64.7 ‰and − 54.4‰), with an average of − 61.1 ‰. The oxygen 18 variation is between (− 9.53‰ and − 7.81‰) with a mean of -8.85 ‰; the isotopic signatures show clearly that this water has a meteoric origin.
Two water groups can be distinguished from the diagram in Fig. 6. This group was distinguished according to their content in heavy stable isotopes as we see the first group is marked with red circle, and groundwater samples are situated under the global meteoric line (GML); the second group samples were located above the meteoric line, (Tables 7 and 8) which confirm that this waters have undergone a high evaporation during the recharge process.
Groundwater from boreholes in a zone between approximately 966 m and 1222 m elevation is relatively depleted in δ18O and δ2H perhaps reflecting a slug of water recharged during colder climatic conditions (Zell E. Peterman and al, 2016).
In addition, the geochemistry of groundwater indicates that the source of sulfate is the dissolution of gypsum. A significant proportion of the calcium in groundwater is shown to be exchanged for sodium in the aquifer (Kimblin 1995). However, in the more mineralized waters, carbonate minerals are precipitated because of contributions to the groundwater of calcium from dissolution of gypsum.
The high mineralization observed in the well (T, Charef) is due to the long contact with evaporate minerals (Gypsum). The saturation index shows clearly that the exchange between these water and the rock are important, which arise the content of the sulfate in these waters.
Water types and classifications
The Piper trilinear diagram was used for the purpose of characterizing the geochemical types. It allows representation of the cations and anions in a single graph in which major groupings or trends in the data can be discerned visually (Piper 1944). Piper diagram (Fig. 8) for the groundwater samples shows that there are three different types of waters. This mineral composition reflects the geological formations that occur at the depth of origin of the thermal spring water, rather than the surface formations.
Physicochemical ability of groundwater for irrigation
To determine the suitability of water for irrigation, we are based on certain parameters such as sodium absorption ratio (SAR), adjusted (SAR) and SSP (percentage Sodium).
where:
-
Sodium absorption ratio (SAR):
-
Na+, Mg2+, Ca2+ are expressed in meq/l.
Calculation of index provides some important conclusion about the ability of theses water for irrigation purposes. The results (Fig. 9) show that all groundwater samples have a SAR < 9, which indicate that Sodium hazard of water is low (Burger and Čelková 2003). This result confirms that groundwater is suitable for irrigation purposes.
Saturation index (SI)
This parameter indicates the geochemical process occurring in the aquifers. The saturation index is a useful quantity to determine whether the water is saturated, undersaturated, or supersaturated with respect to the given mineral:
SI = 0 | IAP = Ksp | → | saturated (in equilibrium) |
SI < 0 | IAP < Ksp | → | undersaturated |
SI > 0 | IAP > Ksp | → | supersaturated |
The expression of the result is given by the logarithm of the ratio of IAP to K, which is called the saturation index (SI) (Antonio Dematteis, 1995):
SI = log (IAP / K_s).
SI = 0 corresponds to the balance between the mineral and the water sample.
IF > 0 with a condition of oversaturation of the mineral in water.
IF < 0 to a situation of undersaturation.
The chart ISD = f(ISC) (Fig. 10) show water in oversaturation for calcite and dolomite, and this explains a low circulation flow. It indicates also an old age of the groundwater in the region.
The results in (Fig. 11) show an oversaturation of all water points compared to Aragonite, Calcite and Dolomite. In the other hand, these waters are undersaturation compared to the minerals of Anhydrite, Gypsum and Halite.
Base exchange index (IEB)
The exchange index (i.e., b) expresses the cation exchanges that can take place in an aquifer (Schoeller 1962). The cations involved are as follows: calcium, magnesium, sodium and potassium. The direction of cationic exchanges tends to establish the balance between water and the fine fraction. The basic exchange index is given by the formula:
The Fig. 12, shows the variation of IEB index for the groundwater samples.
From the results, we find that the values of I.E.B are greater than 0; with the exception of two points: the source of rock salt and Hamam Echaref whose IEB is less than zero.
The water points which show negative values mean that the ions of Ca + + and Mg + + are exchanged by Na + and K + . On the other hand, the points which have values greater than zero mean an exchange of the ions of Na + and K + against the Ca + + and Mg + + ions. The degree of exchange gives us an idea of the degree of equilibrium of these waters.
Relative age of groundwater
Groundwater samples were classified according to the degree of oxygen depletion and excess in deuterium. Figures 13 and 14 show the relative age of groundwater.
According to the classification, the samples in ascending order are as follows: OSF4; OSF5; DF1; OSF6; Hamam Echaref; Salt rock are the oldest.
The spring source of Rocher de sel with a much depleted isotopic signature indicating an old age. This must be confirmed by dating in Tritium and Carbon 14. This source is the point of emergence of the aquifer system.
- DF4 bis samples; OSF7; OSF3; OSF10; OSF11 have an average relative age.
- Samples DF5bis; OSF1; T; DF4; OSF12; OSF2; OSF8 have a recent relative age.
Groundwater isotopic classification
Groundwater sample was classified into two group in function of their Deuterium excess (Table 9).
The first group, with d less than 10, explain that rainfall origin of this groundwater has been evaporated before infiltration in a warmly climatic condition. The second group, where d is more than 10, indicate a cold climatic condition participate in recharge of groundwater. The second group contain slug of old water, and this may be confirmed by using isotopic datation by carbon 14.
Groundwater area of recharge:
Areas of recharge are calculated for the region, approximately by using the following equation
The altitude calculated varies between 1234 et 1635 m. Fig. 15 shows the altitude of recharge for each boreholes.
Conclusions
The lithologic units in the study area extend from Cretaceous to quaternary. The geologic information’s indicate that the aquifers system is subdivided into four layers, namely, from the bottom to the top, The Barremian, the Albian, the Turonian and the Mio-Plio-Quaternary(MPQ) aquifers. The geochemical analysis of water reveals the existence of five major nature of groundwater in the region of Djelfa: Ca–Cl; Ca-HCO3; Ca-SO4; Mg-HCO3; Na-HCO3. The saturation indices show water in oversaturation ISD > 0 and ISC > 0. The graphic ISD = f(ISC) demonstrate that groundwater of interest is relatively old. Isotopic study shows groundwater has a meteoric origin, and all water are depleted in heavy stable isotopes (18O and 2H); a new relative age has been determined in function of depletion in Oxygen and excess of deuterium. Two group were identified according to d-excess: the first indicate an origin from warmly climatic condition and the second from a cold period. The altitudes of recharge calculated for groundwater of region vary between 1234 and 1635 m. For future management of water, we must take a particular attention to manage better these resources.
Availability of data and material
Data are provided in paper.
References
Ali Rahmani SE, Chibane B, Boucefiane A (2018) Sensitive analysis of ground recharge estimation model, for semiarid areas. Appl Water Sci. 8:193. https://doi.org/10.1007/s13201-018-0840-5
Ali Rahmani Salah Eddine, Chibane Brahim (2017) Measure of natural radioactivity of environmental samples by using a portable Geiger Muller counter. In: 1st International Conference on Radiations and Applications, ICRA-2017, Algiers, November 20–23, 2017,USTHB, Algeria
Bouchareb-Haouchim FZ (1993) Apports de la géothermométrie et des données de forages profonds à l’identification des réservoirs géothermiques de l’Algérie du nord. Application à la région duHodna. Thèse de Magister, U.S.T.H.B, pp 106. (in French language)
Burger F, Čelková A (2003) Salinity and sodicity hazard in water flow processes in the soil. Plant Soil Environ. 49(7):314–320
Chibane B (2010) Hydrogeological and hydrogeochemical Study in semi-arid area: case study of the Djelfa region. Phd Thesis in hydrogeology. FSTGAT, USTHB, Algeria, p. 200 (in French)
Coetsiers M, Kilonzo F, Walraevens K (2008) Hydrochemistry and source of high fluoride in groundwater of the Nairobi area, Kenya /Hydrochimie et origine des fortes concentrations en fluorure dans l’eau souterraine de la région de Nairobi, au Kenya. Hydrol Sci J 53(6):1230–1240. https://doi.org/10.1623/hysj.53.6.1230
Dib H (1985) Thermalisme de l’Est algérien. Thèse de 3ème cycle,Université d’Alger, pp 281. (in French language)
Eichholz G, Poston JW (1998) Principles of Nuclear Radiation Detection, Lewis Publishers Inc 3d Printing, pp 379
Hoque MA, Burgess WG (2012) 14C dating of deep groundwater in the Bengal aquifer system, Bangladesh: implications for aquifer anisotropy, recharge sources and sustainability. J Hydrol 444–445:209–220. https://doi.org/10.1016/j.jhydrol.2012.04.022
Kedaid FZ, Mesbah M (1996) Geochemical approach to the Bouhadjar hydrothermal system (NE Algeria). Geothermics 25(2):249–257
Kimblin RT (1995) The chemistry and origin of groundwater in Triassic sandstone and Quaternary deposits, northwest England and some UK comparisons. J Hydrol. 172(1–4):293–311. https://doi.org/10.1016/0022-1694(95)02705-T
Krishnaraj Srinivasamoorthy, Murugesan Vasanthavigar, Vijayaraghavan K, Sabarathinam Chidambaram, Paluchamy Anandhan, Ramachandran Manivannan (2011) Use of hydrochemistry and stable isotopes as tools for groundwater evolution and contamination investigations. Geosciences. 1(1):16–25. https://doi.org/10.5923/j.geo.20110101.02
Li S-L, Liu C-Q, Li J, Yun-Chao Lang Hu, Ding LL (2010) Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: isotopic and chemical constraints. Chem Geol 277(2010):301–309
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Peterman Zell E, Neymark Leonid A, King-Sharp KJ, Gascoyne Mel (2016) Isotope hydrology of the Chalk River laboratories site Ontario, Canada. Appl Geochem. 66:149–161
Salcedo E, Garrido S, Vicenta M, Martínez M, Ocampo A (2017) Hydrogeochemistry and water-rock interactions in the urban area of Puebla Valley aquifer (Mexico). J. Geochem. Explor. 181:219–235
Saravanan K, Srinivasamoorthy K, Prakash R, Gopinath S, Suma C (2015) An Evaluation of hydrogeochemistry of groundwater in upper vellar sub-basin using mineral stability and solute transport modelling. Aquat. Procedia. 4:1119–1125
Schoeller H (1962) Les eaux souterraines: Hydrologie dynamique et dynamique, recherche, exploitation et évaluation des ressources, édi. Masson ET Cie (in French)
Stallard RF, Edmond JM (1983) Geochemistry of amazon, the influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Thomas Abraham, Tellam John (2006) Modelling of recharge and pollutant fluxes to urban groundwaters. Sci. Total Environm. 360(1–3):158–179
Yangui H, Zouari K, Trabelsi R, Rozanski K (2011) Recharge mode and mineralization of groundwater in a semi-arid region: Sidi Bouzid plain (central Tunisia). Environm Earth Sci. 63(5):969–979. https://doi.org/10.1007/s12665-010-0771-4
Acknowledgements
Authors present their grateful thanks to the Algerian for water company and the Nuclear research center especially to Dr Cherchali Mouhamed EL Houcine, and the National water resources agency for all help given in the realization of this work.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali Rahmani, S.E., Chibane, B. Geochemical assessment of groundwater in semiarid area, case study of the multilayer aquifer in Djelfa, Algeria. Appl Water Sci 12, 59 (2022). https://doi.org/10.1007/s13201-022-01573-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01573-y