Abstract
Deterioration in soil–water environment severely contributed by heavy metal bioavailability and mobility on soil surface and sub-surface due to irrational increase in wastewater discharge and agrochemical activities. Therefore, the feasibility of adsorption characteristics of the soil is paramount in curbing the problem of micropollutant contamination in the farming vicinity. Soil from a farming site in a populated area in Enugu, Nigeria was collected and tested to measure the lead and cadmium contents using atomic absorption spectrophotometer (AAS). The adsorption potency of the ultisol soil was estimated for identifiable physicochemical properties by standard technique. The mean activity concentration of Pb2+ and Cd2+ was 15.68 mg/kg and 3.01 mg/kg. The pH, temperature, metal concentration and contact time adsorptive effect on the Pb2+ and Cd2+ uptake was evaluated by batch adsorption technique. The Langmuir, Freundlich and Temkin models were fitted into equilibrium adsorption data and the calculated results depict a better and satisfactory correlation for Langmuir with higher linear regression coefficients (Pb2+, 0.935 and Cd2+, 0.971). On the basis of sorption capacity mechanism of the soil, pseudo-second-order model best described the kinetics of both metal ions retention process. The results of the present study indicated that the soil being a low cost-effective adsorbent can be utilized to minimize the environmental risk impact of these metal ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Composite structured soil medium constitute the environmental basis for plants and other biomass survival as well as interaction of minerals and organic matter in ionic formation being instigated by various industrial, agricultural and other anthropogenic activities. In this recent economic face and urbanization, the soil surface is being endangered via assimilation or uptake of some hazardous metallic ions that can affect human health inclusively in the food chain.
Among the metallic toxic inorganic micropollutants with unique properties heavy metals are natural elemental constituents of parent materials in rocks and sediments being needed by the soil in trace amount for optimum performance of plant physiological process (Emenike et al. 2016) whereas the non-essential parts are venomous to plants and humans with increased toxicity in water, soil and air accumulations at very low concentrations tolerance management (Ezeudo 2014). They form precipitates or complex ions with other compounds in mobile phase of the soil diffusing into the food chain infusing carcinogenic threat through translocation to plants. The extensive utilization of agrochemical, wastewater irrigation and other anthropogenic sources through agricultural activities have protrude to an unending release of some toxic heavy metals (Sharma et al. 2006) into the environment which is huge global concern in relation to ecology and human health menace (Wuana and Okieimen 2011; Dube et al. 2011; Hegazi 2013; Tang et al. 2009; Raymond et al. 2014). Increasing mobility rate of these metallic ions in the soil can lead to polluted growing crops and plants. Hence, the solubility of heavy metals in deeply farming soil solutions is of increasing apprehension to the populace as a result of physiological negative influence on the plants as regards to food safety issues, potential health risks such as kidney damage, neurological disorder, cancer, fragile bone and other devastating effects on soil ecosystem (Musa et al. 2017). The capacity of the soil to control excessive uptake of the toxic metals to the root and shoot of the plants readily depends on the type, morphology and porosity of the soil. The productivity of heavy metal polluted agricultural soil can be severely affected by inhibiting the rate of photosynthesis in plants yielding poor crop output and also deteriorating the quality of air and water environments (Umeh et al. 2019, Raymond et al. 2014). The studied town is widely known for farming activities especially some places at Nsukka inducing poorly treated wastewater and other soil-enriching practices that keep metallic ions in soluble and mobile states. Very high concentration of lead and cadmium has been reported so far in tropical Ultisol soils in Enugu tropical and subtropical soils (Oluwasola et al. 2019). Several proposed applied treatment techniques to reduce and control the mobility of these heavy metals in the soil solution have been compared and adsorption process have tendency to abstract and retain toxic metallic ions from aqueous media owing to its economic feasibility, accessibility and efficient purification (Ramachandran and Souza 2013).
The ionic retention at the boundary crust between the adsorbate specie and soil adsorbent is due to some forces of attraction and chemical bondings between the two phases (Siti et al. 2013) and is being influenced by some soil properties such as texture, density, pH, organic matter, CEC, type, clay minerals present in the soil. It occurs as a result of existing unsteady residual forces at the external surface of the liquid or solid that attracts and uphold the molecular species which comes in contact with the adsorbing surface liberating energy to the surroundings (Babel and Kurniawan 2003). The deleterious effect and fate of heavy metals to soils in temperate regions (Al-Hamdan and Reddy 2006) have been severally researched on while significant outcome of data analysis on these toxic micropollutants in African tropical soils are minimal.
In this part of African country, massive population growth and development in industries have forcefully contributed to continual use of agricultural lands with high level of health risk heavy metals harbor. The food security issues emanating from heavy metal polluted soil ecosystem are unbearable which require urgent monopoly.
Presently, the studied area entails largely agricultural practices that can build-up unhealthy heavy metals leading to food chain degradation. Therefore, the aim of this research is to determine the mean concentration of lead and cadmium and physicochemical characteristics of tropical ultisol from an intensely agricultural area as to estimate its adsorption potential for these contaminants using sorption models and kinetics.
Materials and methods
Sampling analysis and physicochemical evaluation
The top soil samples were obtained randomly from varying spots during rainy season at Opi in Nsukka town of Enugu State, Nigeria to a depth of 30 cm from the farming location and carefully mixed together in a homogenized setting to achieve a composite sample. Soil sample was pulverized gently with a pestle and mortar after drying the for some hours (72 h) to remove moisture and thereafter, sieved through a 2-mm mesh size sieve before being stored in a closed cylindrical glass sterile container.
The quantitative spectroscopic analysis was adopted for the tropical ultisol characterization. The heavy metal content in the soil sample was evaluated using the atomic absorption spectrophotometer (AAS) after soil digestion using concentrated nitric acid and hydrochloric acid (1:3) (Ure et al. 2003). The method of Bouyoucos hydrometer was used to ascertain the particle size distribution (Bouyoucos 1964). The potentiometric method being stated in literature (Mclean 1982) was applied to determine soil pH in water and KCl. Adopted literature procedures were utilized to discern the Cation exchange capacity (Chapman, 1965), Organic carbon (OC) and organic matter (Nelson and Sommers 1996) in the agricultural tropical soil sample. The colorimetric estimation of K+ and Na+ was performed using Flame photometer. Exchangeable acidity i.e. Al3+, H+ (Mclean 1982) and bases, i.e., Ca2+, Mg2+ (Thomas 1982) were evaluated by titrimetric analysis. The soil textural codification was estimated by calculation (Soil survey, 2006).
Adsorption procedure
The studied heavy metals adsorptions by Enugu soil were determined using an equilibrium batch technique. An exact concentration of lead and cadmium was prepared by dissolution of 1.598 g of Pb(NO3)2 and 2.744 g of Cd(NO3)2 in 1L of distilled water. Some factors affecting the uptake of the adsorbate species by an adsorbent were varied. The effect of initial adsorbate concentration (15, 30, 50, 100, 150 mg/L), pH (2.0, 4.0, 6.0, 7.0, 9.0), temperature (303, 308, 318, 323, 333 K) and contact time (5, 15, 30, 50, 60 min) were verified. The sorption studies were carried out by adding 0.1 g of soil sample into 20 mL of each metal ion solution and agitated on a circular mechanical magnetic shaker at 120 rpm room temperature for an hour. The equilibrated soil–liquid contents were centrifuged using a centrifuge (US M-512) at 3000 rpm and filtered at the end of the given contact time. At the variation of each equilibrium adsorption parameter, other sorption factors are kept constant at optimal condition. The removal efficiency of the soil adsorbent for Pb2+ and Cd2+ metal ions was determined gravimetrically using Shimadzu atomic absorption spectrophotometer (model AA-7000) at a wavelength of 283.3 nm and 228.8 nm respectively. Each experimental stage was done in triplicate and the mean calculated results were computed. The quantity of lead and cadmium ions removed at equilibrium (mg/g) per unit mass by the tropical soils and the percentage uptake was determined and calculated by the described equation (Shaban et al. 2018):
where Co and Ce are the initial concentrations of metal ions solutions (mg/L) and equilibrium concentrations (mg/L). V is the volume of metal ion—soil solution, while M is the mass of soil adsorbent (g). Ae and Qe are the adsorption efficiency and quantity adsorbed.
Adsorption isotherm
Few sorption models were used to evaluate the equilibrium adsorption processes. Langmuir sorption theory described the homogeneity distribution of adsorbed molecules on sorbent active sites (Mbadcam et al. 2011) and is given by the following linear equation (Langmuir 1918):
where qe (mg/g) and Ce (mg/L) are the quantity of solute adsorbed per unit mass of adsorption and equilibrium solute concentration in the bulk solution. KL (L/g) is constant while qL(mg/g) is monolayer adsorption efficiency. The dimensionless constant, RL provide appropriate articulation of Langmuir isotherm (Unuabonah et al. 2007) expressed as:
The identification of the stated parameters in the above equation has been earlier described in (Kovo et al. 2015) literature.
Freundlich isotherm interprets the multilayer adsorption on heterogeneous adsorbent active sites and is being expressed by this linear equation (Freundlich 1906):
where qe (mg/g) and Ce (mg/L) are the quantity of solute adsorbed per unit mass of adsorption and equilibrium solute concentration in the bulk solution. KF (mg/g) is the isotherm constant and 1/n indicates the intensity of the process of adsorption (Tang et al. 2009).
Temkin isotherm was used to evaluate the level of adsorption temperature and bonding energies of the molecules in layer during sorbate and sorbent interactions and is described by the following equation in its linearized form (Temkin 1940):
where qe (mg/g) and Ce (mg/L) are the quantity of solute adsorbed per unit mass of adsorption and equilibrium solute concentration in the bulk solution. The plot of qe ainst lCe enables to determine A and B.
Kinetic modeling
The kinetics of adsorption mechanism was analyzed by pseudo-first-order, pseudo-second-order and Weber and Morris Diffusion models.
Pseudo-first-order kinetic entails the comparative to the disparity of the adsorption rate between the capacity of adsorption (qe) in stability and the capacity at any time (Ibrahim et al. 2006). The linearized form of equation (Wahba and Zaghloul 2007; Jain et al. 2004) is given by:
where qe and qt (mg/g) are the amount of solute adsorbed per unit mass at equilibrium and at time respectively. K1 (min−1) is the order adsorption constant.
Pseudo-second-order model presumes that the limiting step rate may emanate from adsorbing substance involving valence forces by electrons interchange tween the adsorbent and adsorbate species (Demirbas et al. 2007) and is expressed in linear equation (Debnah and Ghosh 2008) as follows:
K2 (g/mg min) is the pseudo-second-order adsorption constant at equilibrium and h = K2qe2.
Weber and Morris or intra-particle diffusion model was evaluated to determine the speed of diffusion of molecules and is given by the following equation (Igwe and Abia 2007):
where qt is the amount of metal ions adsorbed (mg/g) at time. Kid (mg/g min1/2) is the intra-particle diffusion constant and I is the intercept. The linear plot of qt versus t1/2 shows that during the mechanism of intra-particle diffusion, the exclusive rate-determining step takes place (Guler and Sarioglu 2013).
Results and discussion
Soil sample characterization
The physicochemical properties of a tropical soil from deeply cultivating location are presented in Table 1. The soil sample is classified as sandy loam with slightly acidic pH value in aqueous solution and metals exist as free ionic species or as soluble organometals and are more bioavailable. The organic matter content (OM) and the cation exchange capacity (CEC) of the agricultural soil are high which ensure a dependable heavy metal adsorption capacity. Acidity level of the soil is attributed to the organic content as a result of biological activities and agrochemical processes through metallic complexion (Osakwe and Okolie 2015). There is tendency of the soil sample to provide an active site for cation exchange as a result of its high clay particle content.
The mean concentration of lead and cadmium in the agricultural tropical soil are 15.68 mg/kg and 3.01 mg/kg respectively which are relatively high according to world health organization (WHO 2008) standard as a result of unremitting application of phosphatic fertilizer, untreated wastewater irrigation and other agrochemicals at various stages of crop production.
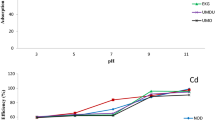
Effect of pH
Solution pH is among the vital parameters that determine the feasibility of sorption process as it readily influences the degree of ionizable metal ions in their specified chemical state (Sheng et al. 2004). The experimental data on the effect of pH relating to percentage removal of Pb2+ and Cd2+ from binary solution by Opi soil are shown in Fig. 1. The level of heavy metals uptake increases with increase in pH with maximum adsorption at pH ≥ 6 for Cd2+ while there is serial retention of Pb2+ onto the adsorbent surface as the pH of the medium increases as a result of sphere coverage complexation and precipitation which enables the adsorptive reactivity of the soil surface (pokrovsky et al. 2012). The deprotonation of the soil coverage site increases at a decreased acidity of soil aqueous solution due to the presence of soluble ligands forming complex compound with the free metals. The removal efficiency of Cd2+ was low in an acidic condition as a result of electrostatic repulsion on the bonding location of the adsorbent site. This present study is comparable with some reported literature (Das et al. 2014).
Effect of metal ion concentration
The effect of concentrations on Pb2+ and Cd2+ adsorption capacity of Opi tropical soil are presented in Fig. 2. Metal ion concentration provides a energy force that overwhelm the extent and effect of transfer of mass resistance of metallic ions between the aqueous and solid phases (Kovo et al. 2015). It was observed from the plot that when the Pb2+ and Cd2+ concentrations increased from 15 to 150 mg/l, the percentage removal of the studied soil decreased which indicates that the insufficient number of active coverage sites of the adsorbent becomes saturated at higher concentrations. Hence, the increase in heavy metal uptake capacity led to an increase increases in metal ions concentration at equilibrium. The soil affinity of the metallic ions is in the order Pb2+ ˃ Cd2+ at concentration of 15, 100 and 150 mg/l. Similar trend has been earlier reported on adsorption of Pb2+ and Cd2+ by lateritic soil (Udoeyo et al. 2010).
Effect of time
The uptake level of Pb2+ and Cd2+ as a function of contact time are shown in Fig. 3. The experimental plot revealed that the metal ions adsorption rate was rapid at the initial stage and gradually reduces as it approaches equilibrium. Therefore, the sorption potential of the studied soil sample increases as the time increases. This implies that during the diffusion process of adsorption, the number of pores on the adsorbent active binding centres increases that sustain the adsorbate species from bulk solution to the adsorbent surface minimizing the mobility and availability of lead and cadmium ions in soil aqueous media. Furthermore, the Opi ultisol has been severely undergoing agricultural practices which activated an increased organic matter and cation exchange capacity contents (Table 1) leading to formation of stable coating as the soil ages. The physicochemical constituents of the soil contributed immensely to its metal ion adsorption efficiency by exposing the available binding sites of the adsorbent as time increases. Some related studies (Mbadcam et al. 2011; Umeh et al. 2019; Olaofe et al. 2015) observed similar trend with this present study.
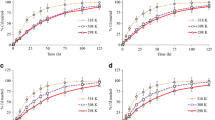
Effect of temperature
The experimental data relating to the varying effect of temperature on adsorption efficiency of Cd2+ and Pb2+ from solution onto Opi soil are shown in Fig. 4. As observed, an increased temperature from 303 to 333 K gave a notifying increase in metal ions adsorption. It is evident that the kinetic energy of the mobile adsorbate molecules in solution are activated as the temperature rises approaching the pore enlarged adsorbent centre faster to be adsorbed. The removal efficiency of the heavy metal ions is in this array Pb2+ ˃ Cd2+. Similar study has been documented in literature (Oluwasola et al. 2019; Nappadol and Pongsakom 2014).
Adsorption isotherm analysis
The equilibrium relationship between the concentration of lead and cadmium adsorbates and the soil adsorbent coverage in solution at a given operational units were assessed by Langmuir, Freundlich, and Temkin isotherms. The studied isotherms provided an insight in optimization of the ultisol adsorbent of its affinity for the metal ions adsorption in addition to the analyzable physicochemical properties of the studied soil. The parameters of the equilibrium isotherms are illustrated in Table 2. A good and satisfactory description for the pd2+ and Cd2+ adsorption efficiency of the Opi tropical ultisol was attested by Langmuir isotherm as shown in Figs. 5 and 6 followed by Temkin isotherms with high correlation value, R2 of 0.935 (Pb2+) and 0.971 (Cd2+). This implies lead and cadmium monolayer coverage applicability and evenly distribution of bonding energies on the studied soil surface during adsorbent—adsorbate interactions. The higher uptake of Pb2+ (28.57) compared to Cd2+ (24.39) regarding maximum adsorption capacity is as a result of increasing electronegativity and decreasing ionic radii of lead. Related studies have been documented (Umeh et al. 2019; Fonseca et al. 2009; Oluwasola et al. 2019). Furthermore, the initial lead and cadmium concentrations (15 mg/L to 150 mg/L) showed ranged values of RL from 0.21- 0.03 (Pb2+) and 0.12–0.01 (Cd2+); thus proposing a favourable adsorption (0 < RL < 1) for both metal ions affinity onto Opi ultisol soil.
Sorption kinetic analysis
The degree of adsorption rate and possible metal ions sorption mechanism on the adsorbent were evaluated by pseudo-first-order, pseudo-second-order and intra-particle diffusion models (Kovo et al. 2015). The relating kinetic parameters of the utilized models are given in Table 3. A better correlation (Fig. 7 and 8) was confirmed by pseudo-second-order theory to the experimental data followed by intra-particle diffusion for Pb2+ and Cd2+ adsorption on the surface of adsorbent specie being identified with high linear regression (R2) values. This suggests the existence of certain boundary layer effect on the soil substrate by which the rate-limiting mechanistic step involving chemical adsorption for Pb2+ and Cd2+ removal is due to the interaction of physicochemical properties between the adsorbent and adsorbate phases. The calculated qe values were more than the experimental obtained values which additionally specify the acceptable desirable quality of the kinetic theory for adsorption of lead and cadmium onto studied Opi soil.
Conclusion
The adsorption potential of Enugu, Nigerian tropical soil for Pb2+ and Cd2+ from aqueous media was explored. The efficiency of the adsorption for the selected studied ultisol for the metal ions increases with increasing operational varying parameters. The maximum retention was attained at contact time of 60 mins which was chosen for sorption equilibrium. Amidst the tested isotherms, Langmuir theory gave a satisfactory and better fit to Pb2+ and Cd2+ experimental plot and data which confirm a homogeneous soil adsorbent surface for the heavy metals uptake. Pseudo-second-order and intra-particle models gave a similar and good conformation for the sorption mechanism of Pb2+ because both models emphasized on rate-limiting process for adsorbent–adsorbate physicochemical interactions while the abstraction capacity for Cd2+ was best suited to pseudo-second-order kinetic. Hence, the studied Enugu, Nigerian tropical agricultural soil can be useful as a low-cost effective adsorbent to control Pb2+ and Cd2+ effects in soil–water ecosystem and as such reducing the environmental human health risk.
References
Al-Hamdan AZ, Reddy KR (2006) Adsorption of heavy metals in glacial till soil. Geotech Geol Eng 24:1679–1693
Babel S, Kurniawan TA (2003).Various treatment technologies to remove arsenic and mercury from contaminated groundwater: an overview In: Proceedings of the First International Symposium on Southeast Asian Water environment, Bangkok, pp 433–440
Bouyoucos GH (1964) A recalibration of the hydrometer for making mechanical analysis of soils. J Agron 43:434
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of soil analysis, part 2. Agronomy No. 9, vol 1965. American Society of Agronomy. Inc., Madison, pp 891–901
Das B, Mondal NK, Bhaumik R, Roy P (2014) Int J Environ Sci Technol 11:1101–1114
Debnah S, Ghosh UC (2008) Kinetics, isotherm and thermodynamics for Cr (III) and Cr(VI) adsorption from aqueous solutions by crystalline hydrous titanium oxide. J Chem Thermodyn 40:67–77
Demirbas E, Kobya M, Senturk E, Ozkan T (2007) Adsorption kinetics for the removal of chromium(VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. J Chem 30(4):533–539
Dube A, Zbytniewski R, Kowalkowski T, Cukrowska E, Buszewski B (2011) Adsorption and migration of heavy metal in soil. Pol J Environ Stud 20:1
Emenike PC, Omole DO, Ngene BU, Tenebe IT (2016) Potentiality of agricultural adsorbent or the sequestering of metal ions from wastewater. Glob J Environ Sci Manage 2(4):411–442
Ezeudo VC (2014) Mobility of heavy metals from the university of nigeria sewage sludge disposal site to the surrounding soils and plants. A Published M.Sc. Thesis submitted to the department of soil science, University of Nigeria, Nsukka, p 104
Fonseca B, Maio H, Quintelas C, Teixeira A, Tavares T (2009) Retention of Cr (VI) and Pb(II) on a loamy sand soil kinetics, equilibria and breakthrough. Chem Eng J 152:212–219
Freundlich HMF (1906) Over the Adsorption in Solution. J Phys Chem 57:385–471
Guler UA, Sarioglu M (2013) Single and binary biosorption of Cu, Ni and methyleneblue by raw and pretreated Spirogyra specie: equilibrium and kinetic modeling. J Environ Chem Eng 1:269–377
Hegazi HA (2013) Removal of heavy metal from wastewater using agriculture and industrial wastes as adsorbents. HBRC Journal 9:276
Ibrahim SC, Hanafiah MAKM, Yahya MZA (2006) Removal of cadmium from aqueous solutions by adsorption onto sugarcane bagasse. Am Eurassian J Agric Environ Sci 1(3):179–184
Igwe JC, Abia AA (2007) Adsorption kinetics and intra particulate diffusivities for bioremediation of Co(II), Fe(II) and Cu(II) ions from waste water using modified and unmodified maize Cob. Int J Phys Sci 2(5):119–127
Jain CK, Singhal DC, Sharma MK (2004) Adsorption of zinc on bed sediment of river Hindo adsorption models and kinetics. J Hazard Mater 114:231–239
Akpomie KG, Dawodu FA, Adebowale KO (2015) Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex Eng J 54(3):757–767
Langmuir I (1918) The adsorption of gases in plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Mbadcam JK, Anagho SG, Nsami JN, Kammegne AM (2011) Kinetic and equilibrium studies of the adsorption of lead (II) ions from aqueous solution onto two cameroon clays: kaolinite and smectite. J Environ Chem Ecotoxicol 3(11):290–297
Mclean EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, vol 1982, 2nd edn. American Society of Agronomy, Madison, pp 199–224
Musa JJ, Mustapha HI, Bala JD, Ibrahim YY, Akos MP, Daniel ES, Oguche FM, Kuti IA (2017) Heavy metals in agricultural soils in Nigeria: a review. Arid Zone J Eng Technol Environ 13(5):593–603
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Page AL (ed) Methods of soil analysis, part 2. Chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison
Noppadol S, Pongsakorn P (2014) Adsorption behaviour of heavy metals on various soils. Pol J Environ Stud 23(3):853–865
Olaofe O, Olagboye SA, Akanji PS, Adamolugbe EY, Fowowe OT, Olaniyi AA (2015) Kinetic studies of adsorption of heavy metals on clays. Int J Chem 7(1):48
Oluwasola HO, Asegbeloyin JN, Ochonogor AE, Ani JU, Ibeji CU, Oyeka EE (2019) Cadmium and lead adsorption capacities of Nigerian ultisol soil of tropics. Orient J Chem 35(3):1004–1012
Osakwe SA, Okolie LP (2015) Physicochemical characteristics and heavy metals contents in soils and cassava plants from farmlands along a major highway in delta state. Nigeria J Appl Sci Env Manage 19(4):695–704
Pokrovsky OS, Probst A, Leviel E, Liao B (2012) Interactions between cadmium and lead with acidic soils: Experimental evidence of similar adsorption patterns for a wide range of metal concentrations and the implications of metal migration. J Hazard Mater 199–200:358–366
Raymond AW, Felix EO, Rebecca NV (2014) Mixed contaminants interaction in soils: implications for bioavailability, risk assessment and remediation. Afr J Environ Sci Technol 8:691
Ramachandran V, Souza SFD (2013) Adsorption of nickel by Indian soil. J Soil Sci Plant Nutr 13(1):165–173
Shaban M, Abukhadra MR, Rabia M, Abd-Elkader Y, AbdElHalim MR (2018) Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr (VI) contaminants from aqeous solutions: kinetic and equilibrium studies. Rend Lincei Sci Fisiche e Naturali 29:141
Sharma RK, Agrawal M, Marshall F (2006) Heavy metals contamination in vegetable grown in wastewater irrigated areas of Varanasi, India. Bull Environ Contam Toxicol 77:312–318
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zincand nickel by marine algal biomass: characterization of biosorptive capacity andinvestigation of mechanisms. J Colloid Interface Sci 275(1):131–141
Siti NAA, Mohd HSI, Md LK, Shamsul I (2013) Adsorption process of heavy metals by low-cost adsorbent: a review. World Appl Sci J 28(11):1518–1530
Soil Survey Staff (2006) United States department of agriculture. Nat Resour Conserv Serv 10:331
Tang X, Zhenze L, Yunmin C (2009) Adsorption behavior of Zn(II) on calcinated Chinese loss. J Hazard Mater 161(824):7
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted ion catalyst. Acta Physicochemistry 12:217–222
Thomas GW (1982) Historical developments in soil chemistry. Ion exchange. Soil Sci Society of American Journal 41:230
Udoeyo FF, Robert B, Hilary I, Sun Young B (2010) Imo lateritic soil as a sorbent for heavy metals. Int J Res Rev Appl Sci 4(1):34–37
Umeh C, Asegbeloyin JN, Akpomie KG, Oyeka EE, Ochonogor AE (2020) Adsorption properties of tropical soils from Awka North Anambra Nigeria for lead and cadmium ions from aqueous media. Chem Afr 3:199–210
Unuabonah EI, Adebowale KO, Olu-Owolabi BI (2007) Kinetic and thermodynamic studies of the adsorption of Lead(II) ions on to phosphate modified kaolinite clay. J Hazard Mater 144:386–395
Ure AM, Quevauviller PH, Muntau H, Griepink B (2003) Speciation of heavy metals in soils and sediments. An account of improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European Communities. Int J Environ Anal Chem 51:135
Wahba MM, Zaghloul AM (2007) Adsorption characteristics of some heavy metals by some soil minerals. J Appl Sci Res 3:421–426
WHO (2008) Permissible limits of heavy metals in soil and plants, (Genava: World Health Organization), Switzerland
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Netw 40(26):4
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umeh, T.C., Nduka, J.K. & Akpomie, K.G. Kinetics and isotherm modeling of Pb(II) and Cd(II) sequestration from polluted water onto tropical ultisol obtained from Enugu Nigeria. Appl Water Sci 11, 65 (2021). https://doi.org/10.1007/s13201-021-01402-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01402-8