Abstract
Numerous deleterious impacts of anthropogenic activities on water quality are typically observed in areas bursting with mineral exploitation, agricultural activities, and industrial processes. Therefore, this contribution details the water quality and water origin in selected hand-dug wells of one the most prominent mining areas in Kenya (Kakamega County). The toxicological impacts of drinking water from a mining site may include cancer and genetic aberrations largely because of the toxic effects of waterborne metals including Hg and As. Accordingly, this study focuses primarily on the investigation of heavy metals, essential elements such as Na and K. Heavy metals and essential elements were determined using spectroscopic and titrimetric techniques. The study revealed that mercury (Hg) concentration ranged between 0.00256 and 0.0611 ± 0.00005 mg/L while arsenic (As) concentration ranged from 0.0103 to 0.0119 ± 0.00005 mg/L. The concentration of potassium ranged from 2.53 to 4.08 ± 0.15 mg/L while that of sodium varied from 6.74 to 9.260 ± 0.2 mg/L. Although the concentration of cadmium was lower than that recommended by W.H.O, the concentrations of Hg, Pb, and As in Kakamega waters were higher than the internationally accepted levels. The generally high level of heavy metals in Kakamega bore-hole waters is, therefore, a public health concern that needs immediate intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bioavailability and biotransformation of trace metals in soil is an important factor in the assessment of the harmful effects on natural ecosystems and human health associated with polluted water (Niesiobedzka 2016). Although water is one of the most essential components of life, it is not accessible to a large number of population in the recommended quality (Nkansah et al. 2010). Heavy metal interactions between groundwater and surface water bodies in river valleys are complex and may be influenced by various factors such as topography, geology, climate and the position of the surface water body relative to the groundwater flow systems (Aleksander-Kwaterczak and Ciszewski 2016). Accordingly, it has become necessary to conduct heavy metal studies in order to determine the quality of drinking water from various sources especially bore-hole water near mining sites. The principal focus of this investigation was to determine the health status of such waters in Kakamega metropolis. Consequently, this study mapped out various sites within Kakamega metropolis that are likely to contain high levels of heavy metals and ultimately inform the general public on the harmful implications of consuming water contaminated with heavy metals. Kakamega County in western Kenya is home to mining activities and as a result water poisoning by heavy metals is a possible public health problem.
The origin of heavy metals in soil ecosystems includes both natural and anthropogenic sources (Niesiobedzka 2016). For the natural sources, soil-forming processes have a strong impact on metal concentrations in soils and ground waters (Aleksander-Kwaterczak and Ciszewski 2016; Niesiobedzka 2016). Soils from mining sites interact with natural ground water and thus change the content of metals, macro-ions, suspended matter, oxygen, pH, temperature, and the deposition of fine sediments which ultimately increase the heavy metal content in groundwater (Aleksander-Kwaterczak and Ciszewski 2016). The role of groundwater for a large population of people in the rural and urban communities in Kenya is vital for life. Statistics have shown that a greater majority (47%) of rural households depend on hand-dug well water for domestic use and other uses (Ofelia et al. 2010; WHO 1993). The traditional water source for a large portion of the population in developing countries is groundwater mainly from hand-dug wells. However, since these wells are dug by hand, their use is restricted to areas with soils of high porosity such as clay, sand, gravel, and mixed soils where only small boulders are encountered during digging. The volume of water in the subsurface aquifer of the well acts as a reservoir which recharges the bore-hole periodically. In recent years, widespread reports of heavy metals and other pollutants have increased public concern on the quality of water in hand-dug wells (Douglas et al. 2012a; Hajdu and Slaveykova 2012). Environmental pollution occasioned by toxic metals represents a potential health risk to both human health and natural ecosystems. For instance, it was previously reported in Bangladesh that high levels of natural arsenic in ground water caused harmful effects on natural ecosystems including higher order animals such as man (Uddin and Huda 2011). Arsenic is known to leach into ground water from wood preservatives, pesticides, and sulphite-containing minerals, and mining areas (Aleksander-Kwaterczak and Ciszewski 2016; McGuigan et al. 2010).
Clearly, public concern about environmental geology and heavy metals pollution can be unpredictable because of the influence from numerous activities such as natural and human activities (Aleksander-Kwaterczak and Ciszewski 2016; Niesiobedzka 2016). Environmental health problems are often considered as disease precursors for carcinogens, mutagens, and other waterborne diseases (Harvey 2008). The threat by cancer due to metal ingestion is real and consequently public awareness on the health effects from microbes and toxic trace metals is fundamental (Klitzke et al. 2012; Miyashita et al. 2012).
The toxicological implications of heavy metals
The toxicity of mercury depends on its chemical form. In the older literature, mercury has played an important role in the processing of gold ores (Vallee 1957). Gold processing using mercury involves a process known generally as mercury amalgamation. Though, large mining companies are no longer using this method for gold extraction, the small-scale miners still rely very heavily on mercury amalgamation for their operation. Both short- and long-term oral exposure to inorganic mercury salts can lead to kidney damage, including kidney failure. It can also cause nausea, vomiting, pain, ulceration, and diarrhea (WHO 1980). Toxicity to the brain and nervous system has been reported following large doses of inorganic mercury taken medically (Bernhoft 2012). Arsenic circulates widely in the lithosphere, hydrosphere and biosphere and is a well-established chemically reactive element and affects the physiological and biochemical activities of biological systems which may lead to poisoning, cancer development, and mental problems (McGuigan et al. 2010; Uddin and Huda 2011). Chronic As poisoning is associated with hyperpigmentation and hyperkeratoses on the hands and feet; cancers of the skin, bladder, liver, and kidney (McGuigan et al. 2010). Environmental pollution by toxic metals represents a serious risk for ecosystems and humans (Hajdu and Slaveykova 2012). For instance, it is well-known in the literature that mercury and its various chemical forms, e.g. gaseous elemental Hg and monomethyl Hg, total Hg (THg) and reactive Hg (HgR) cause serious medical problems in contact with the biological system (Douglas et al. 2012b). It is persistent as well as a highly toxic contaminant that occurs ubiquitously in the environment (Bailey et al. 2001). Mercury in its methylated form is a neurotoxin and is usually released into the aquatic environment through anthropogenic activities (Bailey et al. 2001). Exposure to Cadmium in the population is responsible for broad adverse health effects including damage to kidneys, liver, lungs, and bones (Huang et al. 2011). Cadmium can interfere with some of the organism’s enzymatic reactions, substituting zinc and other metals, manifesting in several pathological processes such as renal dysfunction, hypertension, arteriosclerosis, and growth inhibition, damage to the nervous system, bone demineralization, and endocrine disruption (Ofelia et al. 2010).
The research area
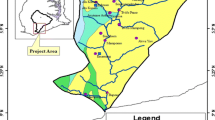
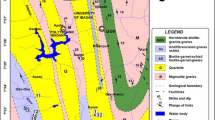
Kakamega metropolis is located in the western part of Kenya (Fig. 1) between 0.2833°N and 34.7500°E, and covers an area of ~ 5 km2. It is home to Kenyans whose economic activities include farming, fishing, and rampant small-scale gold mining. Their sources of drinking water is mainly hand-dug wells whose water quality has not been explored previously. In such a set-up, the quality of water may be affected by rock weathering and waste materials from mining sites that may result in the release of toxic chemicals into the environment, especially into aquatic bodies (Akabzaa 2005). The ills observed through mining activities are rapid loss of farm lands, water in the selected study areas, and heaping of rocks and mine waste around trees; silting of rivers and streams is also evident. Heavy metals from waste rocks that can be leached into water hand-dug wells by similar processes include arsenic, cadmium, mercury, lead, and nickel among others.
This study will provide policy makers and land owners in western Kenya and the Lake Victoria water basin with knowledge of the estimated water quality problems affecting Kakamega metropolis and also serve as a guide for hydrochemistry evaluation of other mining areas in Kenya that share similar characteristics with the area under study. Seven different samples were taken from different sites within Kakamega metropolis and analyzed for physico-chemical properties including pH, electrical conductivity, total dissolved solids, and total water hardness. The metals analyzed included, As, Cd, Pb, Ca, Mg, Na, K while the anions analyzed were \({\text{Cl}}^{ - }\), \({\text{F}}^{ - }\), and \({\text{SO}}_{3}^{2 - }\). The basic objective was to draw conclusions on whether drinking water from hand-dug wells in Kakamega metropolis was within the acceptable limits prescribed by the World Health Organization (WHO) and other international standards such as the European Commission (EC).
Experimental protocol
Materials
All the chemicals used in this study were of analytical grade unless otherwise stated. The reagents and analytical procedure utilized in this study are reported in reference (Zhong-he and Ármannsson 2005).
Analysis of heavy metals
The AAS model, Shimadzu model 6800 with graphite furnace GFA 7000 (Japan) was used for determining the concentration of heavy metals; lead, mercury, arsenic, and cadmium. The procedure for heavy metal analysis is documented elsewhere (Zhong-he and Ármannsson 2005). The machine was operated thus: wavelength and the slit width were 357.9 and 0.2 nm, respectively, flame type: air-acetylene, oxidant flow rate was 1.5 L/min. The sensitivity was 0.055 ppm, detection limit was 0.001 ppm, lamp current was 5 mA and optimum working range of the instrument was 0.001–20.0 ppm.
Titrimetric analysis and pH measurements
The concentration of essential elements including sulphates, total carbonate, F− and Cl− in hand-dug well water was determined automatically by means of a titroline processor using appropriate reagents. The detailed procedure for analysis of these components is described elsewhere (Zhong-he and Ármannsson 2005). The pH of the water samples was determined using a pH meter. The pH probe was rinsed thoroughly using deionized water and calibrated using buffers of pH 7 and 9. Since pH values are temperature dependent, the measurements were carried out at the temperature of the well water. Temperature measurements were taken as soon the water was sampled to investigate if there was any thermal input in the well water. The electrical conductivity of the water samples was measured using a conductivity meter under standard conditions of temperature and pressure. Measurement of conductivity was made immediately at the sample collection site because conductivity conventionally changes with time.
Quality assurance/quality control (QA/QC)
Control sample (bottle water) was analyzed for heavy metals, essential elements, sulphates, carbonates, fluorides, and chlorides prior to the analysis of water sample from hand-dug wells. Standard concentrations were prepared in such a way that it was within the bracket of components in the water sample. This is termed the analysis range. The analyses of samples were conducted in replicates to enhance the validity and reproducibility of the results. To one sample out of 12 samples, a known concentration of the analyte was added and the recovery checked. A recovery of ~ 95% was obtained and this was considered good enough.
Results and discussion
Physico-chemical parameters
Conductivity generally is a measure of free ions in solution; therefore, the higher the number of ions in solution, the higher the conductivity. Clearly, this study has shown that water samples from Muphenji bore-hole (sample 5) hand-dug well had the highest conductivity of 283.0 µs cm−1 while the sample from Iguhu bore-hole (sample 2) had the lowest conductivity of 79.6 µs cm−1. The turbidity levels ranged between 2.34 and 0.88 ntu in the study area. Clearly, the turbidity values are well within the WHO allowable limits for all the hand-dug wells in the study area as reported in Table 1. The anions reported in this work were discussed briefly in our earlier article as critical ligands for metal speciation (Kibet et al. 2016). Their consequent discussion in this study is mainly for quantitative purposes in relation to the water quality of Kakamega metropolis.
Total dissolved solids (TDS) had a bearing on turbidity; generally the samples having higher content of dissolved solids also had higher turbidity. By definition, total dissolved solids comprise of all organic and inorganic matter in a water sample. TDS is not associated with health effects but it is an indication of esthetic characteristics of drinking water. The level of TDS was low in all hand-dug wells except in bore-hole 1 (sample 1—Lusui) which had the highest levels of TDS (142.3) while the sample from bore-hole 2 (sample 2—Iguhu) had the lowest TDS at 39.6. Pure water always contains a small number of molecules that have dissociated into hydrogen ions (H+) and hydroxyl ions. Water samples from Kakamega metropolis were generally neutral and within the permissible pH levels set by WHO, and based on these results alone, are suitable for drinking (WHO 1980). In a previous study, we demonstrated that both pH and cation valency affected the distribution of dissolved and colloidal Pb, As and Hg in water suspensions of a contaminated (Klitzke et al. 2012). Basically, there was very little thermal input in the bore-hole water of Kakamega County considering the temperatures range for the hand-dug wells under study was 0.3 °C. From Table 1, it is clear that the temperature was approximately ambient in all bore-holes.
Essential elements and other waterborne components
In all water samples the level of chloride was found to be within the required level by the world health organization (WHO 1980). Bore-hole 5 had significantly levels of chloride (0.46 mg/L) while bore-hole 4 and 2 had the lowest concentration of 0.30 mg/L (Table 2).
Water sample from bore-hole 5 which was found to have high concentration of chloride was also found to have high conductivity (Table 1). Bore-hole 4 and 2 which had a low concentration of chloride were found to have the lowest conductivity. Potassium varies from 2.53 to 4.08 mg/L while sodium varies from 6.74 to 9.260 mg/L. Clearly from Fig. 2, sodium is the dominant element in all of the Kakamega waters in comparison to potassium. Lusui and Mupenji bore-holes registered the highest levels of sodium (Fig. 2). The same observation can be made for sulphates, total dissolved solids, and electrical conductivity (Tables 1, 2). These bore-holes (Lusui and Mupenji) are, therefore, unique and require further investigation. Furthermore, the mercury levels in these two bore-holes are high as projected in Fig. 3, vide infra. It would, therefore, imply that the water from these bore-holes are not fit for human consumption and must henceforth be abandoned.
All the water samples were found to be within the required amount by W.H.O (250 mg/L). Whereas Lusui, sample 1, had the highest concentration of sulphate at 6.30 mg/L, Shilalunga (sample 6) and Shivikwa (sample 7) had the lowest sulphate concentrations, 0.15 and 0.07 mg/L, respectively. Generally, Kakamega bore-hole water was found to have low fluoride concentrations. Similarly, the chloride levels in all bore-holes were also very low. For instance, the lowest concentration was recorded at Shivikwa (0.24 ± 0.01 mg/L), sample 7, while the highest concentration was noted at Mupenji (0.46 ± 0.01). Accordingly, the fluoride and chloride levels were very low in Kakamega waters. Remarkably, because of the low fluoride levels, fluorosis may not be common in Kakamega County and possibly the larger Western region of Kenya.
According to these findings, it is clear that the concentration of nitrate was generally low and within the W.H.O guidelines; however, sample from bore-hole 1 (Lusui) slightly exceeded the maximum amount of nitrate required by the body; 10.2 mg/L against the WHO recommended level of 10.0 mg/L. The nitrate level in all other boreholes fell below the acceptable WHO limits despite Kakamega County being a sugar farming area where nitrate fertilizers would be expected to leach into the soil and possibly to underground waters. Additionally, the presence of nitrates could be a result of micro-organisms breaking down fertilizers, decaying plants, manures or other organic residues. Usually plants take these nitrates, but sometimes rain or irrigation water can leach them into ground water. High levels of nitrate can cause methemoglobinemia or blue baby syndrome, this is a condition mainly found in infants less than 6 months (Knobeloch et al. 2000). Nonetheless, the nitrate levels in Kakamega waters are within acceptable WHO limits.
Total water hardness
Hardness is defined as a characteristic of water which represents the total concentration of Ca and Mg expressed as their calcium carbonate equivalent. Water hardness may be caused by natural geological process or pollution from industries and commercial operations. The ratio of Ca2+ to Mg2+ in Lusui bore-hole was about 2 (Table 2). This is an interesting result which may suggest that most of the calcium in Lusui waters may be due to maize and tea farming in the upper areas of Lusui (Nandi County). Another remarkable result was observed in Shivikwa borehole, where Mg was twice the concentration of Ca. Although it is expected that Ca should dominate the water because of application of calcium-based fertilizers such as calcium ammonium nitrate and lime in sugar farms, this was not so in Shivikwa waters (sample 7). Large concentrations of Mg may, therefore, be attributed to a geological source containing soil minerals rich in magnesium. Generally, apart from Lusui bore-hole which had the highest concentration of Ca (50.6 ± 1.5 mg/L), all other boreholes contained low concentrations of calcium in comparison to Mg levels. Ideally, Kakamega waters have significantly high concentrations of Mg.
Heavy metals
Heavy metals occur naturally in the ecosystem with significant variations in concentration. In modern times, anthropogenic sources of heavy metals, with significant grave pollution effects have been introduced to the ecosystem (Wojtkowska et al. 2016). In this study, four types of heavy metals including Hg, As, Pb, and Cd are reported. It is clear from the results presented in Fig. 3 that the levels of Hg are higher than those recommended by international agencies in all boreholes (WHO 1980). The range of mercury levels in Kakamega waters lies between 0.0025 ± 0.00001 mg/L in bore-hole 6 (Ikolomani 2) to 0.061 ± 0.0005 mg/L in bore-hole 1 (Lusui). These results, therefore, show that although other species have acceptable levels in Kakamega waters, the high levels of mercury in all the boreholes renders the water unfit for domestic use. This observation is by far the most critical recommendation in this study based on the permissible levels reported in Table 3.
The concentration of arsenic (As) in bore-holes ranged from 0.012 ± 0.0001 to 0.019 ± 0.0001 mg/L. Approximately 5% of the boreholes had arsenic concentration slightly in excess of the WHO recommendations of 0.01 mg/L (WHO 1993). This low percentage suggests that arsenic presently poses only limited potential physiological problem to the use of groundwater for drinking purposes in Kakamega metropolis. The concentration of As is generally very low in most boreholes, and may safely be taken to be below the detection limit of the instrument (Table 4).
Conclusion
It is evident from this study that the concentrations of mercury, lead, and arsenic in most bore-holes are generally in excess of the levels recommended by W.H.O. The elevated concentrations of these metals in the underground waters of western Kenya renders the water unfit for domestic use. These findings, especially on mercury, point to an emerging serious public health problems owing to the use of mercury contaminated waters in Kakamega metropolis and possibly its environs. The concentration of essential elements was found to be within the acceptable limits as recommended by the World Health Organization. Fluoride and nitrate concentrations for instance were low in all the hand-dug wells sampled. Therefore, health problems such as fluorosis and blue baby syndrome may not be common in Kakamega metropolis and generally in various parts of Western Kenya.
References
Akabzaa TM (2005) Impact of mining activities on water in the vicinity of the Obuasi Mine. W Afr J Appl Ecol 79:377–379
Aleksander-Kwaterczak A, Ciszewski D (2016) Pollutant dispersal in groundwater and sediments of gaining and losing river reaches affected by metal mining. Environ Earth Sci 75(95):1–15. https://doi.org/10.1007/s12665-015-4859-8
Bailey EA, Gray JE, Hines ME (2001) Mercury transformations in soils near mercury mines in Alaska. Mater Geoenviron 48(1):212–218
Bernhoft RA (2012) Mercury Toxicity and Treatment: A Review of the Literature. J Environ Public Health. https://doi.org/10.1155/2012/460508
Douglas TA, Loseto LL, Macdonald RW, Outridge P, Dommergue A, Poulain A, Zdanowicz CM (2012a) The fate of mercury in Arctic terrestrial and aquatic ecosystems, a review. Environ Chem 9:321–3551
Douglas TA, Loseto LL, Macdonald RW, Outridge P, Dommergue DA, Poulain A, Christian MZ (2012b) The fate of mercury in Arctic terrestrial and aquatic ecosystems, a review. Environ Chem 9:349–358. https://doi.org/10.1071/EN11140
Hajdu R, Slaveykova VI (2012) Cd and Pb removal from contaminated environment by metal resistant bacterium Cupriavidus metallidurans CH34: importance of the complexation and competition effects. Environ Chem 9:389–398
Harvey AP (2008) Environmental sanitation crisis: more than just a health issue. Environ Health Insights 2:77–81
Huang M, Choi S, Kim D, Kim D, Bae H, Yu S, Park J (2011) Evaluation of factors associated with cadmium exposure and kidney function in the general population. Wiley Periodicals, Inc., New York, pp 563–570
Kibet JK, Munyonge DK, Limo SC, Chemutai LC (2016) The geochemical speciation of Hand-dug well water of Kakamega County, Kenya. Am J Appl Chem 4(2):40–49. https://doi.org/10.11648/j.ajac.20160402.12
Klitzke S, Lang F, Kirby J, Lombi E, Hamon R (2012) Lead, antimony and arsenic in dissolved and colloidal fractions from an amended shooting-range soil as characterised by multi-stage tangential ultrafiltration and centrifugation. Environ Chem 9:462–473. https://doi.org/10.1071/EN12010
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspect 108(7):675–678
McGuigan CF, Hamula CLA, Huang S, Gabos S, Le XC (2010) A review on arsenic concentrations in Canadian drinking water. Environ Rev 18:291–307. https://doi.org/10.1139/A10-012
Miyashita S, Fujiwara S, Tsuzuki M, Kaise T (2012) Cyanobacteria produce arsenosugars. Environ Chem 9:474–484. https://doi.org/10.1071/EN12061
Niesiobedzka K (2016) Mobile forms and migration ability of Cu, Pb and Zn in forestry system in Poland. Environ Earth Sci 75(122):1–8. https://doi.org/10.1007/s12665-015-4821-9
Nkansah MA, Boadi NO, Badu M (2010) Assessment of the quality of water from Hand-Dug wells in Ghana. Environ Health Insights 4:7–12
Ofelia DH, Angel JG, Dailos G, Gonzalo L, Enrique GM, Carmen R, Arturo H (2010) Accumulation of toxic metals (Pb and Cd) in the sea urchin Diadema aff. antillarum Philippi, 1845, in an Oceanic Island (Tenerife, Canary Islands). Environ Toxicol 25:227–233
Uddin R, Huda NH (2011) Arsenic poisoning in Bangladesh. Oman Med J 26(3):207. https://doi.org/10.5001/omj.2011.51
Vallee BL (1957) Mercury and its chemical significance. IArch Ind Health 16:147
WHO (1980) Recommended heaith-based limits in occupational exposure to trace metals. Technical Report Series, Viena, p 647
WHO (1993) Guidelines for drinking water quality. Revision of the 1984 guidelines. WHO, Geneva
Wojtkowska M, Bogacki J, Witeska A (2016) Assessment of the hazard posed by metal forms in water and sediments. Sci Total Environ 551–552:387–392. https://doi.org/10.1016/j.scitotenv.2016.01.073
Zhong-he P, Ármannsson H (2005) Analytical procedures and quality assurance for geothermal water chemistry. United Nations University Geothermal Training Programme, Reykjavik
Acknowledgements
The authors wish to thank the National Government Chemist for allowing us to use their GFAAS instrument to quantify the heavy metals reported in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Christine, A.A., Kibet, J.K., Kiprop, A.K. et al. The assessment of bore-hole water quality of Kakamega County, Kenya. Appl Water Sci 8, 47 (2018). https://doi.org/10.1007/s13201-018-0688-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0688-8