Abstract
The present study deals with the groundwater quality with respect to F− in the Mandavi Taluka of Surat city with an objective to analyze the spatial variability of ground water quality parameter. A total 57 representative groundwater samples from different bore wells and hand pumps were collected during pre-monsoon. Samples were analyzed for various physiochemical parameters including fluoride. GIS technique is adopted to prepare DEM and spatial distribution map of fluoride to represent fluoride concentration in the study area. Results obtained from analysis with GIS mapping reveal that fluoride in the study is mainly attributed to geogenic source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the earth’s most precious and threatened resources whereas health totally depends on it. Thus, we need to both protect and enhance water for health. Groundwater is a major source of water supply for drinking, irrigation and animal husbandry in many countries, especially in arid and semi-arid regions. It is also a vital element of groundwater-dependent ecosystems such as wetlands. Regular and systematic monitoring of groundwater resources is necessary for its effective management to support the water needs of the environment and its citizens.
Industrialization and urbanization in Gujarat from last two to three decades has experienced revolutionary rapid industrial growth. Due to this urbanization, groundwater resources in many countries are coming under increasing threat from growing demands, and contamination. In addition to the various available sources, groundwater forms the major source of drinking water in the rural areas of most of the developing nations in the world. Fluoride (F−) is regarded as an essential trace element, primarily because of its benefits to dental health and its suggested role in maintaining the integrity of bone. The presence of fluoride in drinking water has both positive and negative effects on human health. A small concentration of F− is essential for normal mineralization of bones and the formation of dental enamel. However, the excess concentration F− in groundwater causes an adverse impact on human health. Large groups of people suffer from fluorosis due to intake of F− concentrations above 1 mg/l which may cause dental fluorosis, stiff and brittle bone/joints, deformities in knees; crippling fluorosis; bones finally paralyzed resulting in inability to walk or stand straight and intake of F− concentrations above 3.0 mg/l which may cause skeletal fluorosis.
Traditionally, excessive fluoride has been connected with high intake of fluoride through drinking water and food, but water and food take up fluoride from soil and accumulate it in human body finally through food web; unbalance of fluoride in the human body can cause diseases of teeth and bones which lead to irreversible and permanent damage. In the Indian context, the fluoride is dissolved in groundwater mainly from geological sources. Fluoride epidemic has been reported mostly in granite and gneissic geological formation of different states in India.
The high fluorides occur in top aquifer system and have reached to endemic level in most of the states. In early (1986), fluorosis was reported only in 13 states of India, in 1992 it was 15, in 2002 it was 17 and now it is more than 19, indicating that endemic fluorosis has emerged as one of the most alarming public health problems in the country. At present 62 million people, including 6 million children suffer from fluorosis because of consuming fluoride-contaminated water. Certain blocks of higher instances of fluoride (greater than 1.2 mg/l) have been observed in Mandvi Taluka, Surat. To find out the extent of fluoride contamination in drinking water and to find out the fluoride exposure dose in the study area (Mandvi Taluka, Surat), an extensive study was accomplished by estimating fluoride level in drinking water with respect to BIS and WHO standards.
Materials and methods
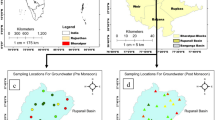
Mandvi is a Taluka in Surat District of Gujarat State, India and lies on the banks of the Tapti River. It is 62 km from Surat and is bounded by Vyara Taluka towards South, Valod Taluka towards South, Bardoli Taluka towards west, Mangrol Taluka towards North. The latitude and longitude of Mandvi Taluka are 21.15°N 73.18°E and having geographical area 829.02 sq. km. The major aquifers in the district are formed by alluvium and Deccan Trap basalt with tertiary formations occupying a small patch. It comprises rocks like Basalt, Rhyolites, Dolerite/Basalt dyke, Laterite, Argillaceous limestone and clay containing nummulites and clay, friable sandstone, pebbly sandstone, the conglomerate which marked as one of the fluoride-rich areas in Mandvi, Surat due to the occurrence of various rock types including fluoride-bearing minerals. The layers of alluvium deposits in the eastern part of the district are weathered and fractured basalt form aquifers. Figure 1 shows sample locations over the geology map of the study area.
The area comes under semi-arid region having tropical and sub-tropical steppe climate with an average annual rainfall of 1475.5 mm and the temperature varies from 30 to 42 °C. The Population of Mandvi is 1, 85,911 approximately. Areas with semi-arid climate, crystalline igneous rocks and alkaline soils are mostly affected (Handa 1975). The origin of fluoride in groundwater is through weathering of alkali, igneous and sedimentary rocks. The common fluoride-bearing minerals are Fluorspar (CaF2), Cryolite (Na3AlF6), Fluor-apatite (Ca3(PO4)2Ca (FCl)2). Fluorite (CaF2) is the principle carrier of fluoride and is found in granite, granite gneisses and pegmatite (Deshmukh et al. 1995; Rao 2009; Rizwan and Gurdeep 2013).
Moreover, there are major excavation projects of minerals (i.e. Lignite, Bauxite, Fluorspar, Limestone, Bentonite, Manganese, Silica sand and Ball clay) near Tadkeshwar in Mandvi. Apart from natural sources, a considerable amount of fluoride may be contributed due to anthropogenic activities like seepage-free circulation of water caused by rainfall and/or irrigation in the weathered products dissolves and leaches the minerals, contributing fluoride to the groundwater (Shaji et al. 2007; Rao 2009).
Analysis of samples
To estimate the fluoride contamination in groundwater at Mandvi Taluka, Surat samples were collected from 57 different locations (Hand pump and Bore well) during summer season (pre-monsoon). The sampling sites are selected to have a fairly comprehensive picture of the fluoride-contaminated locations and fluoride intensity in groundwater. Care was taken to collect subsequent samples from same locations. The samples were taken using acid-washed plastic container to avoid unpredictable changes in characteristic as per standard procedures (APHA 1995). Details of sampling locations along with their latitude and longitude were fixed using a hand-held GPS. Hydrogen ion concentration (pH), temperature and electrical conductivity (EC) were determined on the site only using portable instrument, respectively. F− by SPANDS method, while ions such as Ca2+, Mg2+, HCO3 − using titration method and TDS using gravimetric method by the standard procedures (APHA 1995).
Results and discussions
Fluorine is the lightest halogen group and reactive chemical element and therefore, it is not found as fluorine in the environment. It is important and its extremely high electro-negativity makes it highly reactive and therefore it occurs in a number of naturally combined forms being the most electronegative of all elements (Srivastava and Lohani 2015). It has a strong tendency to acquire a negative charge and forms fluoride ion (F−) in solution. F− has the same charge and nearly the same radius as of the hydroxide ions and may replace each other in mineral structure. Fluoride ions, thus, form mineral complexes with a number of cation and some common mineral species of low solubility contain F− (Hem 1989). The chief source of fluoride in groundwater is fluoride-bearing minerals [i.e., Fluorspar (Fluorite), Apatite (Fluorapatite) and Phosphorite] that exist in rocks and soils.
The weathering and aqueous leaching processes that occur in soils play an important role in determining the amounts of fluoride that reaches groundwater. The various factors that govern the release of fluoride into the water from fluoride-bearing minerals are the chemical composition of the water, the presence and accessibility of fluoride minerals to water, and the contact time between the source mineral and water. Overall water quality (e.g., pH, alkalinity, hardness, and ionic strength) also plays an important role by influencing mineral solubility, complexation and sorption/exchange reactions (Apambire et al. 1997; Meenakshi et al. 2004; Raju et al. 2009). Hence during the chemical reaction, fluoride can easily replace OH− ions in many rock-forming minerals.
During the process of chemical weathering, dissolution of fluoride species in the natural water is controlled by calcium and governed by thermodynamic principles. The calcium ion activity in the natural environment is determined mainly by carbonate ions, which forms insoluble calcite. Water moving through the ground will react to varying degrees with the surrounding minerals (and other components), and it is these rock–water interactions that give the water its characteristic chemistry.
The silicate minerals that comprise most rocks do not react readily with most groundwaters. On the other hand, carbonate minerals do react quite readily with water, and they play an important role in the evolution of many groundwaters. Since carbonates are present in many different types of rock, including most sedimentary rocks, and even some igneous and metamorphic rocks, carbonate chemistry is relevant to the evolution of most groundwaters. The equilibrium constant with respect to calcite can be expressed as:
Above Eq. (1) shows the main mechanism for the dissolution of calcite reaction with carbon dioxide and water, to produce the hydrogen ions (acidic conditions), where ‘K’ represents the solubility product constant and the “a” represents the activity of the ions. Groundwaters that are primarily controlled by carbonate reactions will have relatively high calcium and bicarbonate contents, and, if the rock includes dolerites, then it may also have quite high magnesium levels. Similarly for the solubility of fluorite, the following expression has been used:
(Brown and Roberson 1977).
Further, groundwaters in contact with calcite and fluorite develop equilibrium reactions with both the solid phases which in turn form the following combined mass law equation relating both the solute species. Thus, the following equation explains the saturation of groundwater with respect to calcite and fluorite (Handa 1975; Rango et al. 2008).
Since KCaF2 → CaCO3 is a constant, any change in concentration will be accompanied by a corresponding change in F− concentration indicating a positive correlation between these variables. It has been observed that groundwaters having a high concentration of fluoride when compared with standards are mostly alkaline (WHO 1984; Meenakshi et al. 2004; Rizwan and Gurdeep 2013) and have residual alkalinity, i.e., alkalinity in excess of calcium and magnesium. It is clear that if pH is constant, the activity of fluoride is directly proportional to HCO3 − abundance. This relationship is independent of calcium because of the low solubility product of CaF2. Although the formation of other ions pairs was not taken into consideration for computation of activity of calcium ions, preliminary calculations showed that a small fraction of calcium ions are tied up as complexes and therefore do not affect the broad conclusions drawn above. However, the fluoride content of groundwaters is low in many samples which is due to the areas coming under humid parts which dilutes the concentrations in total dissolved solids as indicated by electrical conductivity values (Table 1).
In groundwater, the solubility of fluoride varies from one rock formation to other. Under normal temperature and pressure conditions, most of the fluorine-bearing minerals are sparingly soluble in water. However, under certain physico-chemical conditions, dissolution activity may become faster (Vikas et al. 2009); however, in alkaline medium, it is desorbed and thus alkaline pH is more favorable for fluoride dissolution; activity while, in acidic medium (low pH) fluoride is adsorbed in clays (Saxena and Ahmed 2003; Raju et al. 2009; Rizwan and Gurdeep 2013). However, it is observed that 76.3% of samples are alkaline in nature and also have a positive correlation (r = 0.27) between pH and fluoride which may favor the solubility of fluorine-bearing minerals in the study area. Minerals which have the greatest effect on the hydro-geochemistry of fluoride are fluorite, apatite, mica, amphiboles, certain clays and villiamite (Raju et al. 2009; Rango et al. 2008). Even taking into consideration aquifers containing fluorine-bearing minerals, water rarely contain more than 8–10 mg/l, due to the very low solubility of F-bearing minerals (Rango et al. 2008). Although CaF2 is one of the major sources of fluoride, its solubility in fresh water and its dissociation rate are very low (Rango et al. 2008; Saxena and Ahmed 2001).
The present study includes chemical composition of groundwaters from confined aquifers which shows that the fluoride content is below 1.1 mg/l and generally below 0.5 mg/l (Handa 1975). A statistical study of the inter-relationship between fluoride and calcium concentrations for low fluoride range (Table 2, Fig. 1) showed a poor coefficient of correlation (dolerites/basalt dykes, rhyolites n = 12 and r = − 0.08) but data presented in Fig. 2 suggest that many of the waters may be at or approaching equilibrium with respect to fluorite. Similarly, in the case of calcium and bicarbonates ions, the correlation coefficients were found to be of low values (dolerites/basalt dykes, rhyolites n = 12 and r = + 0.06, Table 2 the reason is same as above). A study has also been carried out to find the correlation of bicarbonates to the square of the fluoride and it was found that the values do not show much significant variation with respect to the correlation of bicarbonates and fluoride, i.e., showing poor correlation as above. Moreover, the occurrence of low concentration of F− in groundwaters in many sample areas is may be due to the absence of fluoride-bearing magmatic solutions or fluoride-containing minerals in the strata through which groundwater is circulating. It could also be due to rapid fresh water exchange, with the result that the normal process of concentration through evaporation or evapotranspiration is not effective in raising fluoride content of the groundwaters (Handa 1975). Whereas, for high fluoride range, the coefficient of correlation is moderately good and high for the fluoride and calcium concentrations (dolerites/basalt dykes, rhyolites n = 22 and r = − 0.26) and also for the fluoride and bicarbonates (dolerites/basalt dykes, rhyolites n = 22 and r = + 0.70, Table 1, Fig. 2c).
Correlation graphs of some hydro chemical parameters of groundwater in Mandvi Taluka, Surat district. a Fluoride vs. pH diagram showing a positive correlation. b Fluoride vs. calcium significant negative correlation c Fluoride vs. bicarbonate showing a linear positive correlation; and d Fluoride vs. magnesium significant negative correlation
Decreasing calcium concentrations are found under alkaline conditions with a corresponding rise in Na. Therefore, fluoride can accumulate in water if soils and groundwater are low in calcium. In the present case, a broad negative correlation is seen between calcium, magnesium and fluoride (Fig. 2b, d). Table 1 shows the analytical data for the groundwater samples from Mandvi Taluka, Surat.
Evapotranspiration is one of the important reasons for the increase of pH and various constituents which ultimately results in the increase of alkalinity i.e bicarbonates (Subba Rao 2003). Moreover, from Fig. 2a, b, it is observed that a positive correlation is found between HCO3 −and fluoride which indicates that as pH increases alkalinity increases, and which will ultimately increases the fluoride concentration in groundwater samples due to the weathering actions taking place at that particular area. Also, semi-arid climatic conditions lead to increased alkalinity of soil and groundwater. Moreover, the aqueous ionic concentrations of groundwater also appear to have influenced the solubility behavior of fluoride. It has been observed that the bicarbonate type of groundwater dominates the study area. The following are correlation graphs of some hydrochemical parameters of groundwater in Mandvi Taluka, Surat district (Fig. 2).
Geographic information technology (GIS)
GIS is an effective tool for groundwater quality mapping and essential for monitoring the environmental change detection. GIS has been used in the map classification of groundwater quality, based on correlating fluoride ion (F−) values with some aquifer characteristics or land use and land cover (Asadi et al. 2007). Other studies have used GIS as a database system to prepare maps of water quality according to concentration values of different chemical constituents. In such studies, GIS is utilized to locate groundwater quality zones suitable for different usages such as irrigation and domestic (Yammani 2007). Babiker et al. (2007) proposed a GIS-based groundwater quality index method which synthesizes different available water quality data by indexing them numerically relative to the WHO standards. The use of GIS technology has greatly simplified the assessment of natural resources and environmental concerns, including groundwater.
In groundwater studies, GIS is commonly used for site suitability analysis, managing site inventory data, estimation of groundwater vulnerability to contamination, groundwater flow modeling, modeling solute transport and leaching, and integrating groundwater quality assessment models with spatial data to create spatial decision support systems (Barber et al. 1996). A GIS-based study was carried out by Barber et al. (1996) to determine the impact of urbanization on groundwater quality in relation to land-use changes. Nas and Berktay (2010) have mapped urban groundwater quality in Konya, Turkey, using GIS. For any city, a groundwater quality map is important to evaluate the water safeness for drinking and irrigation purposes and also as a precautionary indication of potential environmental health problems.
Moreover, for estimation of groundwater quality of unsampled locations, spatial interpolation is required with a satisfying level of accuracy. Interpolation is based on the principle of spatial auto-correlation or spatial interdependence, which measures the degree of relationship between near and distance points. Spatial auto-correlation determines if values are interrelated.
Depending upon interpolation techniques, there are two categories deterministic and geo-statistical. Deterministic interpolation technique creates surfaces based on the measured points or mathematical formulas, such as inverse distance weighting (IDW), and kriging for spatial data sets. Inverse distance weighting (IDW) method is based on the extent of the similarity of the cells. The IDW function is used when the set of points is dense enough to capture the extent of local surface variation needed for analysis. IDW determines cell values using a linear-weighted combination set of sample points. The weight assigned is a function of the distance of an input point from the output cell location. The greater the distance, the less influence the cell has on the output value. However, the geochemical data in the study area are non-stationary, because many of the closely located points have values drastically different from each other. Because of this reason, IDW method has been used for interpretation of data points instead of kriging to generate maps of continuous maps of geochemical parameters. Figure 3 shows Digital Elevation Model of Mandvi Taluka, Surat. Digital elevation model and geology of study area shows that there is higher elevation towards the northeastern side with some slope and basaltic dykes towards the western and eastern part of the study area.
Figure 4 shows the spatial distribution of fluoride for Mandvi Taluka, Surat. The spatial variations of fluoride ion during the pre-monsoon shows that lower concentrations are distributed along major parts of the study area. The dark pink color shows the fluoride concentration greater than 1 mg/l. Whereas, higher concentrations (> 1.5 mg/l, light pink and blue color) of F− are noted in the south-eastern and north western part of the study area where the geology of the study area is complex comprising of the basalt, dolerites, rhyolites, basalt, gabbro, Quartzite and fluvial nature of the soil. A major patch of F− with concentrations ranging between 0.17 and 0.98 mg/l (i.e. < 1 mg/l, orange and yellow color) are noted in the central, eastern and some western part of the study area.
Conclusion
The main reason for fluoride enrichment in groundwater is considered to be the existence of fluoride-bearing minerals in the host rocks and their interaction with water. The key chemical processes responsible for mobility and transport of fluoride into groundwater are decomposition, dissociation, and dissolution of fluoride-rich rock minerals such as basalt, dolerites, rhyolites, basalt dykes present in the study area. The high concentration of fluoride in groundwater may also be due to the chemical weathering and/or aqueous leaching process under semiarid conditions with relatively high alkalinity, low calcium content and long residence time of interactions. The spatial distribution map of fluoride content in groundwater is very much helpful for the future prediction trend and comparative study over the period of time.
References
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis and health implications of fluoriferous groundwater in the upper regions of Ghana. Environ Geol 33:13–24. doi:10.1007/s002540050221
APHA (American Public Health Association) (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC, p 1467
Asadi SS, Vuppala P, Reddy MA (2007) Remote sensing and GIS techniques for evaluation of groundwater quality in municipal corporation of Hyderabad (Zone-V), India. Int J Environ Res Public Health 4(1):45–52. doi:10.3390/ijerph2007010008
Babiker IS, Mohamed AM, Hiyama T (2007) Assessing groundwater quality using GIS. Water Resour Manag 21(4):699–715. doi:10.1007/s11269-006-9059-6
Barber C, Otto CJ, Bates LE, Taylor KJ (1996) Evaluation of the relationship between land-use changes and groundwater quality in a water-supply catchment, using GIS technology: the Gwelup Wellfield, Western Australia. J Hydrogeol 4(1):6–19. doi:10.1007/s100400050078
BIS (1991) Indian Standard: Drinking water–specification, IS: 105000 (Reaffirmed 1993). Bureau of Indian Standards (BIS), New Delhi
Brown DW, Roberson CE (1977) Solubility of natural fluorite at at 25 °C. USGS J Res 5(4):509–517
Deshmukh AN, Wadaskar PM, Malpe DB (1995) Fluoride in environment: a review. Gondwana Geol Mag 9:1–20
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 13:275–281. doi:10.1111/j.1745-6584.1975.tb03086.x
Hem JD (1989) Study and interpretation of the chemical characteristics of natural water. Water supply paper 2254, 3rd edn. US Geological survey, Washington, DC
Meenakshi, Garg VKM, Kavita R, Malik A (2004) Groundwater quality in some villages of Haryana, India: focus on fluoride and fluorosis. J Hazard Mater 106:85–97. doi:10.1016/j.jhazmat.2003.09.007
Nas B, Berktay A (2010) Groundwater quality mapping in urban groundwater using GIS. Environ Monitor Assess 160(1–4):215–227
Raju N, Dey S, Das K (2009) Fluoride contamination in ground waters of Sonbhadra District, Uttar Pradesh, India. Curr Sci 96(7):979–985
Rango T, Bianchini G, Beccaluva L, Ayenew T, Colombani N (2008) Hydrogeochemical study in the Main Ethiopian Rift: new insights to the source and enrichment mechanism of fluoride. Environ Geol 58:109–118
Rao S (2009) Fluoride in groundwater, Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 152:47–60. doi:10.1007/s10661-008-0295-5
Rizwan R, Gurdeep S (2013) Ground water quality status with respect to fluoride concentration in industrial area of Angul District Orissa, India. Indian J Sci Res Technol 1:54–61 (ISSN:-2321-9262)
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water-rock interaction study. Environ Geol 40:1084–1087
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736. doi:10.1007/s00254-002-0672-2
Shaji E, Bindu JV, Thambi DS (2007) High fluoride in ground-water of Palghat District, Kerala. Curr Sci 92(2):240–245
Srivastava A, Lohani M (2015) Fluorine, a dreaded element: a review on occurrence of fluorine in environment and its standard methods of analysis. Int J Environ Res Dev 5(1):7–21 (ISSN 2249-3131)
Subba Rao N (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh, India. Hydrol Sci J 48(5):835–847. doi:10.1623/hysj.48.5.835.51449
Vikas C, Kushwaha RK, Pandit MK (2009) Hydrochemical status of groundwater in District Ajmer (NW India) with reference to fluoride distribution. J Geol Soc India 73:773–784
WHO (1984) Guidelines for drinking water quality, values 3; drinking water quality control in small community supplies. WHO, Geneva, p 212
Yammani S (2007) Groundwater quality suitable zones identification: application of GIS, Chittoor area, Andhra Pradesh, India. Environ Geol 53(1):201–210. doi:10.1007/s00254-006-0634-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Prajapati, M., Jariwala, N. & Agnihotri, P. Spatial distribution of groundwater quality with special emphasis on fluoride of Mandvi Taluka, Surat, Gujarat, India. Appl Water Sci 7, 4735–4742 (2017). https://doi.org/10.1007/s13201-017-0636-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0636-z