Abstract
Currently, the scientific community is keenly working on environmental-friendly processes for the production of clean energy and sustainable development. The study was conducted to cultivate microalgae in raw institutional wastewater for water treatment, enriched production of biomass and CO2 sequestration. The strains which were used in this study are Scenedesmus sp. and Chlorella sp. which were isolated from Kallar Kahar Lake, Pakistan. Both strains were cultivated in synthetic growth medium (Bold’s Basal Medium) to enhance biomass production. Afterward, microalgae cultures were inoculated in wastewater sample in mixotrophic mode under ambient conditions. The impurities in wastewater were successfully removed from the original sample by the 7th day of operation. COD 95%, nitrate 99.7% and phosphate 80.5% were removed by applying Scenedesmus sp. Meanwhile, Chlorella sp. reduced 84.86% COD, 98.2% nitrate and 70% phosphate, respectively. Interestingly, sulfates were removed from wastewater completely by both strains. Besides being useful in wastewater remediation, these microalgae strains were subsequently harvested for lipid extraction and potential biofuel production was determined. Therefore, the applied method is an environmentally safe, cost-effective and alternative technology for wastewater treatment. Furthermore, the achieved biomass through this process can be used for the production of biofuels.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The demand for energy and clean water is increasing day by day due to the rapid increase in population, industrialization and urbanization. The basic source of the energy is fossil fuels, which are exhaustible and not easily replenished on a human time scale. The consumption of fossil fuels is a major cause of emissions of harmful gases and a distinct thread to climate change. To meet the increasing energy demands as well as keeping in view the environmental protection, there is a need to find out eco-friendly renewable energy sources for sustainable development. Among the renewable sources, biomass production and utilization for biofuel generation is the most attractive technology. Biofuel such as biodiesel is a biodegradable and nontoxic source of fuels. It is being produced from variety of biomass including soybean, sunflower, coconut, canola, palm oil and waste oil; however, its production on large scale is not sustainable (Mostafa et al. 2012). The algae are one of the strong contenders for producing biodiesel. The algae have been grown successfully in different synthetic mediums as well as in different types of wastewater; hence, the water can also be treated in different stages via algae cultivation. The idea of algae cultivation in wastewater is a hybrid approach for the treatment of wastewater, CO2 utilization and production of biomass for biofuels.
Wastewater treatment is a multistage process to purify wastewater before it enters into the natural water body. The purpose of treatment is removal or at least maximize the reduction of organic matter, solids, nutrients or other pollutants through algae cultivation (Buljan et al. 2011). Currently in Pakistan, there is a trend of discharging untreated wastewater (having agricultural, municipal or industrial origin) directly into the nearby water bodies, which significantly alters their physical, chemical and biological nature. There are several issues; in particular, eutrophication, oxygen depletion, increase in turbidity, habitat and aquatic life loss are associated with excessive nutrient loading, which is courtesy of untreated wastewater disposal. More recently, there are some industries that have established wastewater treatment plants on site, which mostly work on the activated sludge method due to its reliability (Irfan 2009). Traditional wastewater treatment methods can be broadly categorized into physical, chemical and biological, such as sedimentation, chlorination and activated sludge. The removal efficiency of most of them are high; however, these conventional methods are not considered as sustainable, thereby presenting a need for a cost-effective and sustainable method.
According to the literature, microalgae is a strong contender for biofuel generation as it can be grown successfully in different synthetic mediums as well as different types of wastewater. Wastewater can also be treated by microalgae cultivation. Previous studies have proved that microalgae utilizes nutrients from wastewater for biomass production which can be used for further production of several useful products such as biodiesel, ethanol, methanol, biomolecules, hydrogen, fertilizer and carotenoids. Hence, treating wastewater by microalgae is a zero waste, environmentally friendly approach and cost-effective, as useful by-products can potentially offset the cost. Microalgae is, hence, a bioremediation agent which is currently being deployed in many facilities. However, recent studies have shown that microalgae potential in wastewater treatment is much wider than its current use. This has been supported by a report published by the National Renewable Energy Laboratory (NREL) which stated that under a controlled environment, microalgae are capable of producing 40% surplus oil for biodiesel per unit area of land as compared to crops such as palm seed or sunflower. Microalgae are being cultivated for the generation of biomass for biofuels; however, microalgae require nutrients to grow and with growing water scarcity issues there is a need for an economical growth medium. With that being said, wastewater can be the best option to cultivate microalgae for biomass, since wastewater contains nutrients and water which can be reclaimed after treatment with microalgae (Woertz 2007). Microalgae growth in wastewater has been indicated as a cost-effective process. Literature depicts that cultivation of microalgae in a controlled environment requires low initial capital, operational cost and low technical costs. It also states that low land-cost areas enriched with sunlight and warm temperature will be advantageous for microalgae-based wastewater system (Woertz 2007; Yun et al. 1997).
Apart from water treatment, microalgae are also major contributors to CO2 sequestration. Emission of CO2 is the main cause of global warming. Microalgae use CO2, NO2 and SO2 as nutrients for their growth. CO2 in flue gas also did not have any inhibitory effect on the algal growth (Yun et al. 1997).
In this study, the isolated algae strains (Scenedesmus sp. and Chlorella sp.) were cultivated in raw institutional wastewater for simultaneous wastewater treatment, CO2 sequestration and biomass harvesting. The aim of the work was to determine the treated water quality, CO2 intake and the lipid contents in biomass. Furthermore, FTIR analysis was carried out on algal biomass to justify its potential of being a feedstock for biofuel (biodiesel) prospects.
Materials and methods
Synthetic medium preparation
Bold’s Basal Medium (BBM) was used as a synthetic growth medium which is ideally used for culturing a wide variety of freshwater algae strains. Having a pH of 6.6, it contained the following chemical composition according to Leslie and Summerell (Leslie and Summerell 2006): K2HPO4 (75 mg/L), MgSO4 (75 mg/L), NaCl (25 mg/L), CaCl2 (25 mg/L), NaNO3 (250 mg/L), KH2PO4 (105 mg/L), FeCl3 (0.194 g/L), MnCl2 (0.082 g/L), CoCl2 (0.16 g/L), Na2MoO4·2H2O (0.008 g/L), and ZnCl2 (0.005 g/L).
Isolation of local microalgae strains
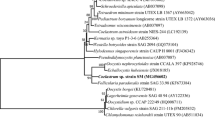
Local microalgae strains were obtained from Kallar Kahar Lake, located in Jhelum, Pakistan, and were isolated and preserved in the Biofuel Lab at Centre for Advance Studies in Energy (CAS-EN), NUST (Pakistan). These strains were revived prior to isolation in BBM at ambient temperature (20 ± 3 °C) with constant CO2 supply (0.04% v/v) at an average flow rate of 0.3 L/min using an aquarium air pump. Illumination was provided via natural sunlight, and the pH was maintained at 7.0. After the revival, serial dilution and agar plating techniques were performed until unialgal cultures were obtained (CSIRO 2013). The resulting isolated strains of Scenedesmus sp. and Chlorella sp. were identified under light microscope (40×, 100×) on the basis of their morphological and other cellular features by comparing with previous studies (Ponnuswamy et al. 2013; Van Vuuren 2006). These strains were then enriched in 10 ml BBM to increase cell concentration. It should be noted that, before the experiments, the BBM medium was first purged with nitrogen gas for a duration of 3 min to remove the dissolved gases and ensure that the initial dissolved CO2 concentration is zero. This is a significant point and is important for CO2 sequestration.
Inoculum preparation and maintenance
Isolated green microalgae were inoculated at 10% (V inoculum/V media) in 250 ml Erlenmeyer flasks containing 100 ml BBM media. These culture flasks were incubated on a shaker (150 rpm) under ambient conditions of (20 ± 3 °C) temperature and natural sunlight at alternating light–dark photoperiod (14 h:10 h) for 20–30 days (Wang et al. 2010). Furthermore subculturing was performed by inoculating 50 μl of algae cultures onto test tubes containing solidified BBM slants with 1.5% (w/v) of agar powder. These test tubes were also incubated at the above conditions for 2–3 weeks during which growth appeared on slants (Prabakaran and Ravindran 2012).
Characteristics of wastewater
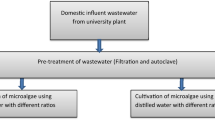
Raw institutional wastewater from the primary septic tank located in Environmental Sciences and Engineering Department, NUST, Pakistan, was used. The representative wastewater sample was grayish black in color and was collected by taking measured volumes of wastewater after every hour and mixing them together. Different physiochemical parameters were evaluated using standard protocols described by the American Public Health Association (APHA) (Eaton 2005). Among the parameters, nitrate nitrogen (NO3-N) was determined using UV–visible spectrophotometry, while phosphate phosphorus (PO −34 -P) and sulfates (SO −24 ) were quantified using spectrophotometer Hach DR/2010. COD was determined using closed reflux method, while turbidity, DO and pH were evaluated using turbidity, DO and pH portable meters, respectively.
Cultivation in wastewater
10% (V inoculum/V media) microalgae cultures were inoculated in reagent bottles containing 1L wastewater samples, respectively. Just like the BBM media, the wastewater samples were first purged with nitrogen gas for 3 min to remove absorbed gases prior to inoculation. The cultures were illuminated under natural sunlight (14 h:10 h) (light–dark photoperiod) at an ambient temperature range of (24 ± 4 °C). CO2 was provided continuously by aquarium air pumps and pH of all cultures was maintained at 7.0–8.0. The incubation period was about 12 days during which microalgae growth parameters as well as wastewater parameters were analyzed.
Analysis of microalgae growth
Samples were taken from the inoculated wastewater on a daily basis for cell count (CC), which was measured using a hemocytometer (Castellanos 2013), whereas dry biomass weight and chlorophyll a were determined after every 3 days. Chlorophyll a (mg/L) was determined spectrophotometrically after extraction by 90% acetone (Arar 1997). Dry biomass weight (g/L) was calculated by filtering 10 ml microalgae culture samples through pre-heated, pre-weighed glass microfiber filters (Whatman GF/C, 47 mm). After filtration, the filters containing microalgae suspension were dried at 103 °C to a constant weight, cooled in a desiccator and weighed on an electronic balance (Irving and Allen 2011).
Growth kinetic parameters
Dry biomass values (g/L) from the exponential growth phase were used for conducting the growth kinetics study. The specific growth rate (µ, day−1) was calculated using Eq. (1), where X 2 and X 1 are the dry biomass weight (g/L) at time t 2 and t 1, respectively.
The maximum specific growth rate (µ max, day−1) was determined from the different µ values calculated, while the maximum biomass obtained was designated as X max (g/L) (de Morais and Costa 2007). Cell doubling time (t d, day) was estimated using Eq. (2), while productivity (P, g/L/day) was calculated using Eq. (3) (Sankar et al. 2011):
where ‘X t ’ is the biomass concentration at time ‘t’, while ‘X o’ is the initial biomass concentration at inoculation time t o. P max (g/L/day) was designated to the maximum productivity. Carbon dioxide uptake rate \(\left( {P_{{{\text{CO}}_{ 2} }} } \right)\) (g/L/day) was measured using Eq. (4) (Elvira-antonio and Ruí 2013), whereas ‘P’ is the productivity calculated above in Eq. (3).
Lipid analysis
The protocol by Bligh and Dyer (1959) was used to extract lipids from microalgae biomass. Microalgae culture was centrifuged (4000g, 10 min) to obtain wet pellet which was mixed in distilled water, methanol and chloroform (4:5:10 v/v) and left overnight on a shaker at moderate speed. The following day, distilled water and chloroform (1:1 v/v) were introduced into the sample and allowed to mix for 5–6 h. Finally the sample was centrifuged (4000g, 10 min) to produce a biphasic layer in which the bottom layer contained lipids dissolved in chloroform. This layer was extracted using micropipette and dried at 50 °C for 2 h in an oven to obtain the lipid content.
FTIR analysis
Fourier transforms infrared (FTIR) spectroscopy analysis was conducted on dried algal biomass at room temperature using Perkin Elmer Spectrum 100 FTIR spectrophotometer (Ponnuswamy et al. 2013). The algal pellet was obtained via centrifugation (4000g, 10 min) and was placed in an oven at 103 °C to dry to constant weight. The obtained dried algal powder was then blended with potassium bromide (KBr) powder and pressed into tablets before analysis in the wavelength region of 4000–500 cm−1.
Results and discussion
Analysis of raw wastewater
The physiochemical analysis of raw wastewater revealed the presence of color in the sample, which was due to the presence of high level turbidity and other dissolved and undissolved substances that may have also contributed to the odor (Iram et al. 2013). The chemical oxygen demand (COD) level was observed to be slightly above the National Environmental Quality Standards (NEQs) of 150 mg/L (Shams 2000). The overall nutrient load suggested that the wastewater was in low–medium strength range as reported in (Gogate and Pandit 2004). Table 1 shows the initial physiochemical analysis of the collected raw wastewater.
Analysis of algae growth in wastewater
The isolated microalgae strains demonstrated no lag phase, as good adaptability was observed in the wastewater medium since the start. Both strains showed a similar growth trend till 6 days, after which Chlorella sp. displayed a decline while Scenedesmus sp. continued to exhibit increased exponential growth till the 7th day. After 7 days, both strains entered the decline phase as algae cells continued to die off. Growth curves of Chlorella sp. and Scenedesmus sp. in terms of CC (million cells per ml) vs time are shown in Fig. 1.
In this study, the Scenedesmus sp. achieved the maximum production of chlorophyll a (19.04 mg/L), while Chlorella sp. showed the second best results with 13.1 mg/L in wastewater culture on the 8th day. Afterward, both strains showed decline in chlorophyll a content to 8.59 and 8.88 mg/L, respectively, on the 12th day as microalgae cells entered death phase. According to Young et al. (1996), chlorophyll a production shows similar growth trend to cell concentration, i.e., the highest concentration of chlorophyll a will be obtained at the highest cell concentration or at the end of the exponential phase of growth and can be observed in Fig. 2.
Biomass yield is an expression of organic production, as the dry weight of the organic mass produced over a period of time; it is also used to express microalgae growth. The highest dry weight was achieved using Scenedesmus sp. (0.445 g/L) as compared to Chlorella sp. (0.39 g/L) in wastewater medium on the 8th day. Afterward, both strains showed drastic reduction till the 12th day, representing nutrient deficiency in the media. Furthermore, the data showed linear biomass growth, which reflected linear carbon dioxide (CO2) sequestration as algae uses carbon dioxide from air to carry out photosynthesis to produce oxygen and glucose. Greater CO2 uptake demonstrates greater biomass production as an indicator of microalgae growth. The CO2 uptake rates of the selected microalgae strains are presented in Table 2 along with overall growth kinetics and dry weight for both strains (see Fig. 3).
From the growth studies, it is evident that the growth of Scenedesmus sp. is better than that of Chlorella sp. in our wastewater sample. It was also reflected in the overall growth kinetics (Table 2).
FTIR analysis and lipid content of isolated microalgae strains
FTIR Spectroscopy was employed to determine the presence of vibrationally active functional groups (including O–H, N–H, C=O, C–H, CH2, C–O–C and >P = O) in the isolated microalgae strains (Venkatesan et al. 2012). Chlorella sp. and Scenedesmus sp. both showed strong peaks in the range of 2809–3012 and 3300–3560 cm−1, indicating the presence of lipid–carbohydrate and protein–carbohydrate functional groups (Table 3). This, along with peaks in the range of 1000–1200 cm−1, strongly indicated the presence of carbohydrates in Chlorella sp. depicting higher carbohydrate potential as compared to Scenedesmus sp. Both Chlorella sp. and Scenedesmus sp. showed strong peaks at protein amide I band. This band is primarily due to C=O stretching vibrations in amide complexes (Dean et al. 2010). The presence of alcoholic (1260–1350 cm−1), ester (1250–1300 cm−1) and nucleic acid (1191–1356 cm−1) functional groups was observed in Chlorella sp., while the functional group of antioxidant enzymes (1030–1120 cm−1) was observed in both algal strains. Overall, from FTIR analysis, it was observed that Chlorella sp. had higher lipid and carbohydrate content along with nucleic acid in comparison to Scenedesmus sp., making it a better biofuel feedstock, if optimum cultivation and later harvesting conditions are provided.
After the FTIR analysis, the next step was to validate and quantify the lipid content potential of the microalgae strains via experimentation. For this purpose, the lipid content was extracted from the harvested biomass at the exponential growth phase. The results validated the observations of FTIR analysis by indicating that Chlorella sp. have higher lipid content than Scenedesmus sp. in both growth media (Fig. 4). The total lipid percentages were reported to be high, as 45% of pure cultures of Scenedesmus sp. and Chlorella sp. were grown in BBM (Thompson 1996). The experiments were conducted in ambient conditions without optimization, i.e., under lower illumination, temperature and without pure CO2 supplement. The resulting lipid contents of the pure strain cultures grown in BBM were much lower than those previously reported (Prabakaran and Ravindran 2013; Sharma et al. 2016). On the other hand, strains cultivated in institutional wastewater demonstrated higher lipid content under similar ambient conditions. This might be due to the wastewater sample being nitrogen deficient with respect to BBM. When the nitrogen is exhausted from medium culture, the cell nitrogen is used in enzymes and essential cellular structures and a portion of the carbon dioxide content is converted to lipid and carbohydrates, giving higher lipid yields (Kamalanathan et al. 2016). Overall, the results suggest that the strains have the potential of producing higher lipid content in the wastewater treatment application if the process is optimized.
Analysis of treated wastewater sample
The effect of microalgae cultivation on NO3-N, SO −24 and PO −34 -P nutrient stripping was observed. The results indicated a significant reduction in the above nutrients during the course of experimentation. PO −34 -P was removed up to 70 and 80.5% from wastewater on the 7th day by Chlorella sp. and Scenedesmus sp. respectively. It depicted effective uptake of phosphorus to be accumulated and synthesized as polyphosphates in their bodies. Nearly, 50% removal was observed after 3 days by both strains as seen in Fig. 5.
The removal rates for NO3-N (the major inorganic nitrogen form in the influent) in 7 days were 98.2 and 99.7% for Chlorella sp. and Scenedesmus sp., respectively. The results from the experiment showed up to 47% reduction by Chlorella sp. in 4 days, while Scenedesmus sp. achieved 50% removal by the 5th day (Fig. 6). According to Wang et al. (2010), the relative constancy of nitrate uptake by algae might be due to the saturation of assimilators to amino grouping production for entry into nitrogenous metabolism. This homeostasis of nitrogen assimilation enables it to maximize growth, which supports our findings.
SO −24 was removed completely within 7 days by both strains. Being the only form of sulfur source in wastewater medium, eukaryotic microalgae consumed SO −24 to manufacture S-amino acids, sulfo-lipids and other S-containing metabolites during its growth phase (Prieto and Vega 1992).
Besides nutrients, both strains displayed a significant reduction in COD and turbidity levels. The efficiency for turbidity removal was above 90% for both strains. COD ,which represents the organic matter present in wastewater that can be oxidized chemically, was reduced up to 84.86 and 95% by Chlorella sp. and Scenedesmus sp. on the 7th day of cultivation, respectively (Fig. 7). This COD removal was attributed to the attached and suspended growth of the algal biomass on the aeration tube as well as in the mixture. During cultivation, algal cells visibly increased, turning the wastewater color from blackish gray to green, which when filtered produced clear water. The overall reduction in wastewater pollutants within 7 days of incubation is listed in Table 4. DO increased significantly from 3.51 mg/L to 8.65 and 8.5 mg/L in wastewater by Chlorella sp. and Scenedesmus sp., thereby improving the overall water quality and making it acceptable for discharging or reuse purpose according to the National Environmental Quality Standards (NEQs).
Conclusion
Discharged municipal, industrial and agricultural wastewaters contain many organic and inorganic contaminants which stimulate adverse impacts on human health and aquatic life. Treatment of such wastewaters by microalgae is an attractive green option as it can sequester carbon dioxide, reduce nutrient load and generate biomass as feedstock for biofuel production. In this study, Chlorella sp. and Scenedesmus sp. were isolated and grown on raw institutional wastewater for simultaneous nutrient removal and biomass/lipid production. Results indicated significant improvement in water quality within a detention period of 7 days. This treated wastewater can be reused or discharged into the environment, as excessive nutrients and COD were reduced to levels within the limits of the National Environmental Quality Standards (NEQs). Both strains showed amazing growth trends and significant biomass yield. The harvested biomass was checked for the biofuel potential via FTIR analysis, whose results regarding lipid content were validated by performing lipid extraction and quantification. The outcome demonstrated the potential of these local strains as suitable candidates for biodiesel production to combat rising fuel issues, especially Chlorella sp. if favorable environmental conditions are provided. Furthermore, the algal residue left after oil extraction can be utilized for fertilizer or other biofuel production, thereby presenting possibilities for an economical treatment system. With that being said, more research should be conducted to determine the extent of scale-up for this technology and an energy/economics analysis to understand the level of sustainability of the system.
References
Arar EJ (1997) In Vitro determination of chlorophylls a, b, c 1 + c 2 and pheopigments in marine and freshwater algae by visible spectrophotometry adapted by National Exposure Research Laboratory Office of Research and Development U. S. Environmental Protection Agen,” no. September, pp 1–26
Bligh EG, Dyer WJ (1959) Bligh-Dyer method. Can J Biochem Physiol 37(8):911–917
Buljan FSJ, Kral I, Clonfero G (2011) Introduction to treatment of tannery effluents. UNIDO, Vienna
Castellanos CS (2013) Batch and continuous studies of Chlorella vulgaris in photo-bioreactors. In: Electronic thesis and dissertation repository, The University of Western Ontario, paper 1113
CSIRO (2013) Microalgal isolation techniques. [Online]. http://www.marine.csiro.au/. Accessed 15 Sep 2015
de Morais M, Costa J (2007) Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers Manag 48(7):2169–2173
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101(12):4499–4507
Eaton AD (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, D.C
Elvira-antonio N, Ruí A (2013) Effect of nitrogen content and CO2 consumption rate by adding sodium carbonate in the lipid content of Chlorella vulgaris and Neochloris oleoabundans. Int J Environ Prot 3(2):13–19
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8(3):553–597
Iram S, Kanwal S, Ahmad I, Tabassam T, Suthar V, Mahmood-Ul-Hassan M (2013) Assessment of physicochemical parameters of wastewater samples. Environ Monit Assess 185(3):2503–2515
Irfan M (2009) Wastewater treatment in textile, tanneries and electroplating industries especially by activated sludge method—a technical report. J Pakistan Inst Chem Eng XXXVII:4–5
Irving TE, Allen DG (2011) Species and material considerations in the formation and development of microalgal biofilms. Appl Microbiol Biotechnol 92(2):283–294
Kamalanathan M, Pierangelini M, Shearman LA, Gleadow R, Beardall J (2016) Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J Appl Phycol 28(3):1509–1520
Leslie F, Summerell A (2006) Media—recipes and preparation. Fusarium Laboratory Manual, Hoboken, New Jersey
Mostafa SSM, Shalaby EA, Mahmoud GI (2012) Cultivating microalgae in domestic wastewater for biodiesel production. Notulae Sci Biologicae 4(1):56–65
Ponnuswamy I, Madhavan S, Shabudeen S (2013) Isolation and characterization of green microalgae for carbon sequestration, waste water treatment and bio-fuel production. Int J Bio-Sci Bio-Technol 5(2):17–26
Prabakaran P, Ravindran A (2012) Scenedesmus as a potential source of biodiesel among selected microalgae. Curr Sci 102(4):616–619
Prabakaran P, Ravindran D (2013) Selection of microalgae for accumulation of lipid production. Caribb J Sci Technol 1:131–137
Prieto JL, Vega JM (1992) Sulfate uptake in Chlamydomonas reinhardtii. Phyton 32:1989–1992
Sankar V, Daniel DK, Krastanov A (2011) Carbon dioxide fixation by Chlorella minutissima batch cultures in a stirred tank bioreactor. Biotechnol Biotechnol Equip 25(3):2468–2476
Sharma AK, Sahoo PK, Singhal S, Patel A (2016) Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 6(2):116
Shams K (2000) Review on NEQS. In: The gazette of Pakistan, vol 57, no. M, pp 189–194
Thompson GA (1996) Lipids and membrane function in green algae. Biochem Biophys 1306:17–45
Van Vuuren SJ (2006) Easy identification of the most common freshwater algae: a guide for the identification of microscopic algae in South African freshwaters. Resource quality services (RQS), Pretoria, South Africa
Venkatesan S, Pugazhendy K, Sangeetha D, Vasantharaja C, Prabakaran S, Meenambal M (2012) Fourier transform infrared (FT-IR) spectoroscopic analysis of spirulina. Int J Pharm Biol Arch 3(4):969–972
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162(4):1174–1186
Woertz IC (2007) Lipid productivity of algae grown on dairy wastewater as a possible feedstock for biodiesel. California Polytechnic University, San Luis Obispo
Young AJ, Harker M, Tsavalos AJ (1996) Reference. J Ferment Bioeng 82(2):113–118
Yun YS, Lee SB, Park JM, Lee CI, Yang JW (1997) Carbon dioxide fixation by algal cultivation using wastewater nutrients. J Chem Technol Biotechnol 69(4):451–455
Acknowledgements
The authors are grateful to the Biofuel laboratory, Centre for Advanced Studies in Energy (CAS-EN) NUST, for providing funds for this study. The authors are also thankful to the Institute of Environmental Sciences and Engineering (IESE), NUST, Islamabad, Pakistan, for providing laboratory facilities. Without their support, this research could not have progressed well.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ansari, A.A., Khoja, A.H., Nawar, A. et al. Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl Water Sci 7, 4151–4158 (2017). https://doi.org/10.1007/s13201-017-0574-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0574-9