Abstract
Delhi has emerged as one of the greenest capital city of the world. Microbiological assessment of drinking water emphasizes estimation of the hygienic quality of the water sold with reference to community health significance. This study was conducted to evaluate the quality of drinking water sold by roadside vendors in east, west, north and south zones of capital of India. A total number of 36 samples (nine from each zone) were collected as per national guidelines and studied for microbiological assessment. All the drinking water samples were collected in gamma-sterilized bottles and were kept in an ice pack to prevent any significant change in the microbial flora of the samples during the transportation. The water samples were transported to the laboratory in vertical position maintaining the temperature 1–4 °C with ice pack enveloped conditions. Samples were analyzed for total MPN coliform per 100 ml and for the presence and absence of common human pathogenic bacteria such as Escherichia coli, Salmonella, Staphylococcus aureus and Pseudomonas aeruginosa. All the samples were found to be contaminated with coliform organisms in the range of 14 to >1600 per 100 ml of sample. Out of 36 water samples, the occurrence of E. coli was 61 %, Salmonella 25 % S. aureus 14 % and P. aeruginosa 53 % as 22, 9, 5 and 19 samples were found contaminated, respectively. The numbers of coliform bacteria and presence of some common pathogenic bacteria suggested that the quality of drinking water sold by roadside vendors is not within the Indian standard and WHO guidelines laid down for drinking water quality. Hence, there is a vital need to study the root cause in terms of hygiene, sanitation of vendors and source of contamination to prevent waterborne diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In India, the health burden of poor water quality is massive. Assessment of drinking water quality is essential in order to provide quality drinking water intended for human consumption and for all usual domestic purposes. Isolation and enumeration of organisms such as E. coli indicates the presence of fecal contamination. Isolation and identification of other specific pathogens (Salmonella, S. aureus and P. aeruginosa, etc.) in water should be undertaken only for purposes of investigating and controlling outbreaks of disease. Smith et al. (1999) estimated 25–33 % of the global burden of disease can be attributed to environmental risk factors such as contaminated water. Almost 10 % of the global disease burden could be prevented by improving water supply, sanitation, hygiene and management of water resources (WHO 2008). According to Morris and Levine (1995) the annual waterborne disease burden in the United States of America (US) estimated 560,000 people may suffer from moderate-to-severe waterborne infection, and that 7.1 million suffer from a mild-to-moderate waterborne infection each year (Medema 2003). Around 37.7 million Indians are affected by waterborne diseases annually, 1.5 million children are estimated to die of diarrhea alone. Access to safe drinking water is important as a health and development issue at a national, regional and local level. In some regions, it has been shown that investments in water supply and sanitation can yield a net economic benefit, since the reductions in adverse health effects and health care costs outweigh the costs of undertaking the interventions. This is true for major water supply infrastructure investments through to water treatment in the home. Experience has also shown that interventions in improving access to safe water favor the poor in particular, whether in rural or urban areas, and can be an effective part of poverty alleviation strategies. Accessibility and availability of fresh clean water is key to sustainable development and an essential element in health, food production and poverty reduction (Third World Water Forum 2003). According to the WHO, the mortality of water-associated diseases exceeds 5 million people per year. From these, more that 50 % are microbial intestinal infections, with cholera standing out in the first place. Water-related diseases continue to be one of the major health problems globally. Water can be defined as safe if does not cause any significant hazard to health over a lifetime of consumption. Safe drinking water is thus suitable for all purposes, including personal hygiene. Acute microbial diarrheal diseases are a major public health problem in developing countries. People affected by diarrheal diseases are those with the lowest financial resources and poorest hygienic facilities. Children under five, primarily in Asian and African countries, are the most affected by microbial diseases transmitted through water (Seas et al. 2000). The analysis of the microbiological flora of water supplied for drinking purposes shows that Delhi water is biologically contaminated (Singh 2000). The Mega City of Delhi is referred to as the national capital territory of India. Delhi is located at 28°37′N 77°14′E 28.61°N 77.23°E, and lies in Northern India. It has 1485 per sq. km area with a population of nine million. The density of population is 6319/km2. The temperature varies as follows. January 3–15 °C; April–May 27.5–46 °C; July 30–32.5 °C; October below 25 °C. The annual rainfall ranges from 40 to 200 cm. The mega city has a dry winter, hot summer followed by heavy rains (Susheela et al. 1996). The microbiological quality of drinking water is a concern to consumers, water suppliers, regulators and public health authorities. The potential of drinking water to transmit microbial pathogens to great number of people causing subsequent illness is well documented in many countries at all levels of economic development (Dufour et al. 2003).
The most reliable source of drinking water is bottled water which is of good bacteriological quality (Obiri et al. 2003), but it is expensive and thus only within the means of the affluent in the society. While some employ sophisticated techniques such as ozonisation and reverse osmosis, most use ordinary boiling of well water sources and exclusion of particles by waterborne diseases constitutes one of the major public health hazards in developing countries. Worldwide, in 1995, contaminated water and food caused more than 3 millions deaths, of which more than 80 % were among children under age 5 (WHO 1996). From microbiological point of view, the safety of water depends on various aspects from its production to final consumption in such a way so that either any microbial contamination can be prevented or it will be reduced to levels not harmful to health. Water can support the growth of many types of microorganisms. This can be advantageous, for example, the chemical activities of certain strains of yeasts provide us with beer and bread. Many microorganisms are found naturally in fresh and saltwater. These include bacteria, cyanobacteria, protozoa, algae, and tiny animals such as rotifers. These can be important in the food chain that forms the basis of life in the water. For example, the microbes called cyanobacteria can convert the energy of the sun into the energy it needs to live. The plentiful numbers of these organisms in turn are used as food for other life. As well, the growth of some bacteria in contaminated water can help digest the poisons from the water. However, the presence of other disease-causing microbes in water is unhealthy and even life threatening. For example, bacteria that live in the intestinal tracts of humans and other warm-blooded animals, such as E. coli, Salmonella, Shigella and Vibrio, can contaminate water if feces enter the water. Contamination of drinking water with a type of E. coli known as O157:H7 can be fatal. The intestinal tract of warm-blooded animals also contains viruses such as rotavirus, enteroviruses, and coxsackievirus that can contaminate water and cause disease. Protozoans are the other group of microbes of concern in water microbiology. The two protozoa of the most concern are Giardia and Cryptosporidium. They live normally in the intestinal tract of animals such as beaver and deer. Giardia and Cryptosporidium form dormant and hardy forms called cysts during their life cycles. The cyst forms are resistant to chlorine, which is the most popular form of drinking water disinfection, and can pass through the filters used in many water treatment plants. If ingested in drinking water they can cause debilitating and prolonged diarrhea in humans, and can be life threatening to those people with impaired immune systems. Besides the conventional pathogens, which are transmitted by water, several emerging waterborne pathogens have become increasingly important during the last decade or so. Several waterborne diseases are commonly reported from metropolitan cities of India, most likely due to unsatisfactory disinfection of municipal water (Singh 2000). These include Vibrio cholera 0139, toxin producing E. coli especially enterohemorrhagic E. coli (EHEC) (Szewzyk et al. 2000; Sharma et al. 2003), Salmonella typhi the organisms causing typhoid fever (Madigan et al. 1997). Ideally drinking water should not contain any microorganisms known to be pathogenic or any bacteria indicative of fecal pollution (Prasai et al. 2007). Detection of fecal indicator bacteria in drinking water provides a very sensitive method of quality assessment and it is not possible to examine water for every possible pathogen that might be present (WHO 1993). The greatest microbial risks are associated with ingestion of water that is contaminated with human or animal feces (George et al. 2001). An important aspect of water microbiology, particularly for drinking water, is the testing of the water to ensure that it is safe to drink.
The objective of the present study is to evaluate the microbial contamination level both quantitatively and qualitatively from each selected zone of Delhi. The findings will go a long way to help residents make informed choice regarding their drinking water quality.
Materials and methods
Method of collection of water samples
Gamma-irradiated, clean and sterilized bottles (200 ml capacity) were used for sampling of water from different zones of Delhi, India. For dechlorination sodium thiosulphate was added to the clean, dry sampling bottles before gamma sterilization in an amount to provide an approximate concentration of 100 mg/l in the sample. Aseptic conditions were maintained during the collection of samples. Approx. 200 ml of water sample was sampled from each location as per the guidelines given in Indian Standard. The samples were kept in an ice pack to prevent any changes in the microbial flora of the samples during transportation. The water samples were transported to the laboratory in vertical position maintaining the temperature 1–4 °C with ice pack enveloped conditions. Samples were analyzed within 6 h of collection.
Media, chemical and reagents
All the media were procured form Hi-Media Laboratory, Mumbai, India. Procured dehydrated media were used as per the instructions written on the box and growth promotion test of each media carried out before evaluation of samples. Sodium chloride, sodium thiosulphate and other chemicals were of analytical grade. Rabbit plasma, anti-sera O, H, Vi, Gram-stain kit and other reagents were procured from Difco Laboratories.
Enumeration of coliform bacteria (MPN coliform/100 ml)
The most probable number (MPN) technique as per IS1622:1981 was implemented for the enumeration of total coliform. The test procedure included three phases namely presumptive, confirmative and completed phase.
Presumptive test
For each water sample; 5 tubes of each 10, 1, and 0.1 ml were used. 10 ml sample was inoculated in double strength MacConkey broth media and rest 1 and 0.1 ml was inoculated in single strength MacConkey broth media. All the inoculated tubes were incubated at 37 °C for 24–48 h. Tubes showing presence of growth (turbidity) with or without gas were submitted to confirmatory phase.
Confirmative test
All presumptive tubes showing positive results were gently shaken and using micropipette and sterile tips culture were added to BGBL (Brilliant Green Bile Lactose Broth) and incubated at 37 °C for 24–48 h. Formation of gas within the period of 48 h was taken for completed phase. Numbers of positive tubes were recorded.
Completed test
The cultures from the positive tubes were streaked on EMB (Eosin Methylene Blue) agar and these plates were incubated at 37 °C for 24 h in inverted position. Presence of green metallic sheen colonies confirms the presence of E. coli. Some of these colonies from individual plates were transferred on non-selective media nutrient agar slants for further biochemical identification of E. coli. These isolates were further confirmed by Gram’s staining and biochemical tests for E. coli as per IS: 5887. Isolation and identification of Pathogens:
Method of detection of E. coli
Membrane filtration method is also used for the secondary detection of E. coli. 250 ml of water sample was passed through 0.45-µm filter and the filter paper was inoculated in MCB. Confirmatory identification was done by subculturing on Eosin Methylene Blue agar and on MacConkey agar. Plates were observed for characteristic colonies such as pink colonies on MacConkey agar and green metallic sheen colonies on Eosin Methylene blue agar plates. Further confirmation was done by biochemical tests for E. coli as per IS: 5887(part-1) 1976, Reaffirmed 20005.
Evaluation
On the basis of characteristic colonies as observed on selective media and morphological, biochemical evaluation, results are recorded as ‘E. coli Present or Absent/250 ml of Water Sample’.
Positive and negative control
During the experiment, quality control is achieved by running the pure cultures obtained from Culture Collection Centers. During this experiment, E. coli as ‘Positive Control’ and S. aureus as ‘Negative Control’ are used.
Method of detection of Salmonella sp.
250 ml water sample was passed through 0.45-µm filter and the filter paper was inoculated in buffer peptone water and then incubated at 37 °C for 24 h. 0.1 ml of the above enriched sample was inoculated in 10 ml of Rappaport–Vassiliadis (RV) medium and then incubated at 42 °C for 24 h and then were subcultured on the plates brilliant green agar and bismuth sulphide agar. The plates were observed for characteristic colonies such as pink colonies on brilliant green agar and black metallic sheen colonies with H2S on bismuth sulphide agar plates. Further confirmation several biochemical and serological tests were carried out for Salmonella as per IS: 5887(Part-3) 1999, Reaff. 2005.
Serological identification
The detection of the presence of Salmonella O-, Vi- and H-antigens is tested by slide agglutination with the appropriate sera, from pure colonies. If the bacteria have clumped into more or less distinct units with one drop of normal saline solution then the strain is considered auto-agglutinable, and shall not be submitted to the following tests as the detection of the antigens is impossible.
Examination for O-antigens
On using one drop of anti-O serum instead of the saline solution if agglutination occurs, the reaction is considered positive.
Examination for Vi-antigens
On using one drop of anti-Vi serum instead of the saline solution if agglutination occurs, the reaction is considered positive.
Examination for H-antigens
On using one drop of anti-H serum instead of the saline solution if agglutination occurs, the reaction is considered positive.
Evaluation
On the basis of characteristic colonies and biochemical test, results are recorded as ‘Salmonella Present or Absent/250 ml of Water Sample’.
Positive and negative control
During the experiment, quality control is achieved by running simultaneously, Salmonella typhimurium as ‘Positive Control’ and E. coli as ‘Negative Control’.
Method of detection of Pseudomonas aeruginosa
For the detection of Pseudomonas aeruginosa 250 ml water sample was passed through 0.45-µm filter and the filter paper was inoculated in cetrimide broth and then incubated at 37 °C for 48 h, then subcultured on the plates of cetrimide agar. The plates were observed for characteristic green colonies and further confirmation was by Gram’s staining and biochemical test as per IS: 13428:2005 (Annexure-D).
Evaluation
On the basis of characteristic colonies and biochemical test, results are recorded as ‘Pseudomonas aeruginosa Present or Absent/250 ml of Water Sample’.
Positive and negative control
During the experiment, quality control is achieved by running simultaneously, Pseudomonas aeruginosa as ‘Positive Control’ and E. coli as ‘Negative Control’.
Method of detection of Staphylococcus aureus
For the detection of S. aureus 250 ml water sample was passed through 0.45-µm filter and the filter paper was inoculated in cooked medium and then incubated at 37 °C for 24 h. Subcultured on the mannitol salt agar and Baird Parker agar plates. Plates were observed for characteristic colonies such as yellow colonies on mannitol salt agar plates and black colonies on Baird Parker agar plates. Further confirmation was done by Gram’s staining and biochemical test as per IS: 5887(Part-2) 1976, Reaff. 2005.
Evaluation
On the basis of characteristic colonies and biochemical test, results are recorded as ‘S. aureus Present or Absent/250 ml of Water Sample’.
Positive and negative control
During the experiment, quality control is achieved by running simultaneously, the pure cultures of S. aureus as ‘Positive Control’ and E. coli as ‘Negative Control’.
Results and discussion
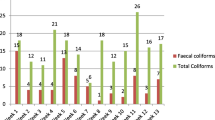
The results of present study including biochemical characteristics of isolated bacterial strains are summarized in Tables 1, 2, 3, 4, 5. A total number of 36 (nine samples per zone) of drinking water were collected from roadside vendors. These samples were analyzed for enumeration of total coliform bacterial population and for the presence and absence of common human pathogenic bacteria such as E. coli, Salmonella, S. aureus and P. aeruginosa. The drinking water resources were severely contaminated in this region. The coliform bacteria were found in all collected samples. The lowest number was 14 and highest number more than 1600 recorded. Presence of MPN coliform indicates that the degree of pollution and sanitary quality of drinking water. Out of 36 water samples, the occurrence of E. coli was 61 %, Salmonella 25 % S. aureus 14 % and P. aeruginosa 53 % as 22, 9, 5 and 19 samples were found contaminated, respectively, (Fig. 1).
For the estimation of MPN coliform, no significant trends were observed in the collected samples from all sites of each zone. However, the number of MPN coliform bacteria was higher in the samples collected from north zone (NDDWS). E. coli were found in each zone. In east Delhi, out of nine samples, seven samples were found to be contaminated with E. coli, i.e., 77 %. However, three samples were found E. coli positive out of total nine samples from west Delhi. Contamination of E. coli in north Delhi samples was same as in east Delhi (77 %). Five samples were found to be contaminated from south zone. Presence of other common pathogenic microorganisms was comparatively less: Salmonella (9 out of 36), S. aureus (5 out of 36) and P. aeruginosa (nineteen out of thirty-six). The variation in contamination depends on the quality of available water and largely depends on the treatment and designed distribution system in the region. The presence of these bacterial strains indicates that the water is contaminated and requires detail study to find out the root cause of general distribution system in the city. Although the sources of contamination are of primary importance for drinking water quality, other climatic and locality factors may also influence the bacterial contamination rates in open water sources. According to WHO (1984) guidelines, the occurrence of pathogens or indicator organisms in ground and surface water sources mainly depends on intrinsic physical and chemical characteristics of the catchment area and the magnitude and range of human activities and animal sources that release pathogens to the environment. The occurrences of these bacteria in drinking water are of primary importance because these organisms generally live together in water than other intestinal pathogens and therefore can be detected easily.
The occurrence of coliform bacteria in water could be due to fecal contamination, i.e., discharge of feces by humus and other animals in water. According to Klein and Casida (1967), coliform may be used as water quality indicator, and if such bacteria are not detectable in 100 ml, the water can be said as potable water. The continuous consumption of such contaminated water may pose serious health risks in local residents of these areas especially in children. The presence of coliforms shows the danger of fecal pollution and consequent hazard of contracting disease through pathogenic organisms. Nonetheless, the disease-causing organisms (pathogens) mostly transmitted via drinking water are predominantly of fecal origin. Coliforms are mostly of human and animal fecal origin hence their increase can be correlated with human and other activities. Raina and Saha (1984) also studied bacteriological parameter and found that water was contaminated with coliforms in their area. Many members of the total coliform group and some so-called fecal coliforms (e.g., species of Klebsiella and Enterobacter) are not specific to feces, and even E. coli has been shown to grow in some natural aquatic environments (Ashbolt et al. 1997; Bermudez and Hazen 1988; Hardina and Fujioka 1991; Niemi et al. 1997). Trabulsi et al. (2002) concluded that typical enteropathogenic E. coli strain is a leading cause of infantile diarrhea in developing countries, whereas they are rare in industrialized countries. Unlike E. coli, humans infected with salmonellae can carry the bacteria in the gut without signs of disease. Infected humans can harbor the bacteria for considerable periods of time. About 5 % of patients clinically cured from typhoid fever remain carriers for months or even years. These people can be chronic holders of the bacterium in the gut, and constitute the main reservoir of the bacteria in the environment (Popoff and Le minor 2005). Staphylococcus aureus was also identified in drinking water samples of various regions. It is a pathogenic bacterium responsible for several issues of severe health problems, e.g., food spoilage, chronic infections, abscesses, wound infection. In general, S. aureus occurs in water containing organic pollutants, i.e., minerals ions and organic matter (Tortora et al. 1988).
In rural areas, people prefer to eliminate soils in open places especially in agriculture fields. In such conditions, there are more possibilities of contamination of open water resources through rainwater runoff mechanism. Vibrios are primarily aquatic bacteria. Species distribution depends on sodium concentration and water temperature. Only serovarieties O1 and O139 are involved in true cholera. Some other serovarieties can cause gastroenteritis, but not cholera. The illness caused by serovarieties O139 and O1 are indistinguishable (Farmer et al. 2005; Ali et al. 2001; Ramamurthy et al. 2003). According to Pujari et al. (2007) the onsite sanitation that is increasingly adopted in India is possibly responsible for high levels of these bacterial contaminations in drinking water sources. However, the biggest problem associated is that the presence of pathogenic bacteria in water is sporadic and levels are low thus the isolation and culture of these bacteria is not very easy task. Safe water demands that water is free from pathogenic bacteria. Total results suggest that due to the lack of awareness of good sanitation and personal hygienic practices, the drinking water sold by roadside hawker has very low nutritional values.
Conclusion
Quality of drinking water is essential for all human beings. Government authorized body exists to provide drinking water to the community of Delhi. Available raw water is treated by several means and supplied. The presence of coliform and other common human pathogenic bacteria indicates that the drinking water sold by roadside vendors is not fit for drinking. It is necessary that drinking water sources should be tested regularly to know whether water is meeting the prescribed standards for drinking or not and, if not, then, the extent of contamination/unacceptability and the follow-up required. Failure to ensure drinking water safety may expose the community to the risk of outbreaks of intestinal and other infectious diseases. Drinking waterborne outbreaks are particularly to be avoided because of their capacity to result in the simultaneous infection of a large number of persons and potentially a high proportion of the community. In addition to fecally borne pathogens, other microbial hazards (toxic Cyanobacteria and Legionella) may be of public health importance under specific circumstances. The authorities and regulatory body ensure the good practices of quality water distribution chain in the city. There is a need to educate all vendors about the possible source of contamination and drinking water distribution system. At the same time there is an urgent need to strengthen/improve the water management chain so that quality of drinking water is ensured in all means. Our findings clearly reveal the deprived quality of water sold in Delhi and may further be used for further long study to validate the data and to reduce the risk in the area.
References
Ali M, Emch M, Yunus M, Sack RB (2001) Are the environmental niches of Vibrio cholerae 0139 Different from those of Vibrio cholerae 01 El Tor? Int J Infect Dis 5:214–219
Ashbolt NJ, Dorsch MR, Cox PT, Banens B (1997) Blooming E. coli, what do they mean? In: Kay D, Fricker C (eds) Coliforms and E. coli. Problems or Solution?. The Royal Society of Chemistry, Cambridge, pp 78–85
Bermudez M, Hazen TC (1988) Phenotypic and genotypic comparison of Escherichia coli from pristine tropical waters. Appl Environ Microbiol 54:979–983
Dufour A, Snozzi M, Koster W, Bartram J, Ronchi EL, Fawtrell (2003) Assessing microbial safety of drinking water, improving approaches and methods. WHO/OECD; p 11
Farmer JJ, Janda JM, Brenner FW, Cameron DN, Birkhead KM (2005) Genus Vibrio. In: Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, vol 2, Part B, pp 494–546
George I, Crop P, Servais P (2001) Use of ß-DGalactosidase and ß-D-Glucuronidase activities for quantitative detection of total and faecal coliforms in wastewater. Can J Microbiol 47:670–675
Hardina CM, Fujioka RS (1991) Soil: the environmental source of Escherichia coli and enterococci in Hawaii’s streams. Environ Toxicol Water Qual 6:185–195
IS 13428 (2005) Detection and enumeration of pseudomonas aeruginosa (Annexure-D)
IS 5403 (1999) Reaff: 2005. Method for yeast and mould count of foodstuffs and animal feeds
IS 5402 (2002) Reaff: 2007. Microbiology- general guidance for the enumeration of microorganisms colony cont technique at 300 C
IS 5401 (Pt-1) (2002) Reaff: 2007 microbiology general guidance for the enumeration of Coliforms
IS 5887 (Pt-1) (1976) Reaff: 2005. Isolation, identification and enumeration of Escherichia coli
IS 5887 (Pt-2) (1976) Reaff: 2005 isolation, identification and enumeration of Staphylococcus aureus and Faecal streptococci
IS 5887 (Pt-3) (1999) Reaff: 2005. General guidance on methods for the detection of Salmonella
IS 5887 (Pt-5) (1976) Reaff: 2005 isolation, identification and enumeration of Vibrio cholera and Vibrio parahaemolyticus
Klein DA, Casida LE (1967) E. coli die out from normal soil as related to nutrient availability and the indigenous microflora. Can J Microbiol 13:1456–1461
Madigan MT, Martinko JM, Parker J (ed) (1997) Brock biology of microorganisms. 8th edn, International edition
Medema, GJ, Payment P, Dufour A, Robertson W, Waite M, Hunter P, Kirby R, Anderson Y (2003) Safe drinking water: an ongoing challenge. In: Assessing microbial safety of drinking water. Improving approaches and method; WHO & OECD, IWA Publishing, London, pp 11–45
Morris RD, Levine R (1995) Estimating the incidence of waterborne infectious disease
Niemi RM, Niemelä SI, Lahti K, Niemi JS (1997) Coliforms and E. coli in finnish surface waters. In: Kay D, Fricker C (eds) Coliforms and E. coli. Problems or Solution?. The Royal Society of Chemistry, Cambridge, pp 112–119
Obiri DK, Okore-hanson A, Jones K (2003) The microbiological quality of drinking water sold on streets in Kumasi, Ghan. Lett Appl Biol 37:334
Popoff MY, Le minor LE (2005) Genus Salmonella. In: Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology, 2nd edn. Springer: New York, vol 2, Part B, pp 764–799
Prasai T, Lekhak B, Joshi DR, Baral P (2007) Microbiological analysis of drinking water of Katmandu Valley. Sci World 5(5):112–114
Pujari PR, Nanoti M, Nitnaware VC, Khare LA, Thacker NP, Kelkar PS (2007) Effect of on-site sanitation on groundwater contamination in basaltic environment: a case study from India. Environ Monit Assess. doi:10.1007/s10661-007-9616-3
Raina VAR, Saha SA (1984) Pollution studies on water quality. Int J Environ Health 26:187–201
Ramamurthy T, Yamasaki S, Takeda Y, Nair GB (2003) Vibrio Cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect 5:329–344
Seas C, Alarcon M, Aragon JC, Beneit S, Quiñonez M, Guerra H, Gotuzzo E (2000) Surveillance of bacterial pathogens associated with acute diarrhea in Lima. Peru Int J Infect Dis 4:96–99
Sharma S, Singh I, Virdi JS (2003) Microbial contamination of various water sources in Delhi. Curr Sci 84(11):1398–1399
Singh S (2000) Isolation of free living Amoebas as a highly sensitive index of water contamination. Water Int 25(3):403–409
Smith KR, Corvalán CF, Tord KT (1999) How much global ill is attributable to environmental factors. Epidemiology 10(5):573–584
Susheela AK, Bhatnagar M, Kumar A (1996) Status of drinking water in the mega city Delhi. In: 22nd WEDC conference, New Delhi, India, pp 299–301
Szewzyk U, Szewzyk R, Manz W, Schleifer KH (2000) Annu Rev Microbiol 54:81–127
Third World Water Forum (2003) Blockade, myth, illusions in development and cooperation. Volume 30, No 1
Tortora GJ, Funke BR, Case CI (1988) Microbiology: an introduction, 3rd edn. Benjamin/Cumming, California
Trabulsi LR, Keller R, Tardelli Gromes TA (2002) Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis 8(5):508–513
WHO (1993) Guidelines for drinking water quality. Vol II–III. World Health Organizations, Geneva
WHO (1996) Fighting disease, fostering development. The World Health Report, Geneva
WHO (World Health Organization) (2008) Guidelines for Drinking-water quality, incorporating 1st and 2nd Addenda, volume 1, recommendations, 3rd ed. WHO; Geneva, Switzerland
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research manuscript has no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chauhan, A., Goyal, P., Varma, A. et al. Microbiological evaluation of drinking water sold by roadside vendors of Delhi, India. Appl Water Sci 7, 1635–1644 (2017). https://doi.org/10.1007/s13201-015-0315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0315-x