Abstract
In the present study, a coagulation process was used to treat paper-recycling wastewater with alum coupled with poly aluminum chloride (PACl) as coagulants. The effect of each four factors, viz. the dosages of alum and PACl, pH and chemical oxygen demand (COD), on the treatment efficiency was investigated. The influence of these four parameters was described using response surface methodology under central composite design. The efficiency of reducing turbidity, COD and the sludge volume index (SVI) were considered the responses. The optimum conditions for high treatment efficiency of paper-recycling wastewater under experimental conditions were reached with numerical optimization of coagulant doses and pH, with 1,550 mg/l alum and 1,314 mg/l PACl and 9.5, respectively, where the values for reduction of 80.02 % in COD, 83.23 % in turbidity, and 140 ml/g in SVI were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pulp and paper mills are among the most important industries in the world, but also some of the biggest polluting agents, discharging a variety of pollutants such as gaseous, liquid and solid wastes into the environment. The pollution of water bodies is of major global concern, because these industries generate large volumes of wastewater, viz. about 80 m3 of wastewater for each ton of pulp produced (Oguz and Keskinler 2008). More than 250 chemicals produced at different stages of paper production have been identified in the effluents (Thompson et al. 2001). According to research reports, samples with biodegradability index (BOD5/COD) smaller than 0.3 are not appropriate for biological degradation (Helble et al. 1999), as for complete biodegradation the effluent must present an index of at least 0.40 (Chamarro et al. 2001).
Paper mill wastewater can cause considerable damage to the recipient waters due to the high chemical oxygen demand (COD) and high toxicity. To minimize the impact of effluents on the environment, several treatment technologies have been employed, although little is known on their efficiency to eliminate the toxicity attributed to the presence of organic compounds. This is mainly due to the fact that it is the type of paper mills (packaging, recycling, kraft) and the water system configuration that determine the COD, toxicity and organic load of the effluent. Therefore, the design and efficiency of wastewater treatments will vary from mill to mill (Latorre et al. 2007). Thompson et al. (2001) reviewed the different types of treatment of pulp and paper mill effluents and indicated effective processes to minimize the discharge of wastewater to the environment. Coagulation is one of the most used water effluent treatments. It employs a cationic metal as a coagulant agent which usually promotes water hydrolysis and the formation of hydrophobic hydroxide compounds with different charges, depending on the solution pH. It may also lead to the formation of polymeric compounds. The coagulants interact with colloidal materials by either charge neutralization or adsorption, leading to coagulation usually followed by sedimentation (Stephenson and Duff 1996). The coagulation effectiveness and cost depend on the coagulant type and concentration, solution pH, ionic strength as well as on both concentration and nature of the organic residues in the effluent (Afzal et al. 2008). The response surface methodology (RSM) is a statistical technique for designing experiments, building models, evaluating the effects of several factors, searching optimum conditions for desirable responses and reducing the number of experiments (Wang et al. 2007). RSM has been proposed to determine the influence of individual factors and their interactive influence. It uses an experimental design such as the central composite design (CCD) in order to fit a modeling by the least squares technique, and the adequacy of the proposed model is then revealed using the diagnostic checking tests (Anderson-Cook et al. 2009).
The main objective of this work was to optimize the coagulation process and investigate the interactive effects of the experimental factors, viz. the coagulant dosages, pH and initial value of COD as process parameters. For this purpose, paper-recycling wastewater was selected as the target to be treated by the coagulation process, optimizing it by RSM under Design-Expert software. The quadratic models obtained were used in a constrained optimization to achieve optimum process conditions for maximum removal efficiencies of turbidity and COD and the lowest sludge volume index (SVI) of the paper-recycling wastewater.
Materials and methods
Analytical methods
The paper-recycling wastewater used in this work was taken from Afrang Paper Manufacture Ltd., Iran. The wastewater samples were characterized according to standard methods for the examination of water and wastewater (APHA 1998) and the results are given in Table 1. Alum and PACl (Merck) were used as coagulants. The initial solution pH of the paper-recycling wastewater was adjusted using 1.0 mol/l sodium hydroxide and 0.5 mol/l sulfuric acid solution. A calorimetric method with closed reflux was developed for the measurement of COD (the Plaintest system, photometer 8000, England) at 600 nm, which was used to measure the absorbance of the COD samples. A pH meter (the Waterproof CyberScan PC 300, Singapore) was used to measure the solution pH. Turbidity was measured by a turbidity meter (the CyberScan TB 1000 Eutech Instruments, Singapore). The SVI was measured using a 1,000 ml Imhoff cone (APHA 1998).
Experimental procedure
The coagulation experiments were performed with 100 ml paper-recycling wastewater, adjusting the initial COD and pH at different values. In a typical run, the paper-recycling wastewater was diluted with tap water to adjust its COD concentration to the desired value. Then different concentrations of coagulants were progressively added. The samples were stirred at 50 rpm (Velp scientific magnetic stirrer) for 2 min to completely dissolve the coagulant. This was followed by a further slow mixing for 20 min at 40 rpm. The flocks formed were allowed to settle for 30 min. After settling, the turbidity and final COD of the supernatant were determined. The remaining portion of the treated wastewater samples was used to determine the SVI.
Experimental design for optimization of parameters
The RSM was applied for developing, improving and optimizing the processes and to evaluate the relative significance of several affecting factors even in the presence of complex interactions. In the present study, the Design- Expert 7.0 (State-Ease, Inc., Minneapolis, MN, USA) software was used for regression and graphical analyses of the obtained data. RSM, as a robust design technology based on the central composite design (CCD), could be applied to the modeling and analysis of multiple parameters. The experimental design had with four different factors: solution pH, initial COD value, alum dosage, PACl dosage. Each of the parameters was coded at five levels: −α, −1, 0, +1 and +α. A CCD involves a two-level factorial (k 2) design, 2 k axial points (denoted by ± α), and n c center points. In this study, the goal was to create a full factorial design at the k = 4 design, completely randomized, in one block with six center points and the axial distance α = 2 for a rotatable design. The range and level of the variables in coded units from the RSM studies are given in Table 2. For statistical calculations, the variable X i was coded as x i, according to the following equation:
where X i is the uncoded value of the independent variable, i, X 0 is the value of X i at the center point of the investigated area and ∆X is the step change. Table 3 shows the coded and uncoded variables for the four experimental variables according to the Eq. (1). To obtain the optimum dosages, initial COD and pH, three dependent parameters were analyzed as response, final COD and turbidity removal efficiencies and SVI value. The quadratic equation model for predicting the optimal conditions can be expressed according to Eq (2) as follows:
where (i) is the linear coefficient, (ii) the quadratic coefficient (β) the regression coefficient, (ij) the interaction coefficient, (k) the number of factors studied and optimized in the experiment and (ε) the random error. The experiments were carried out according to the experimental data sheet (both coded and uncoded design matrices) shown in Table 3, which also lists the response values, i.e., COD removal, turbidity removal and SVI value. The results are further analyzed using Design-Expert Software. The relationship between the four controllable factors (Alum and PACl dosages, pH and initial COD) and the three important operating parameters (COD removal, turbidity removal and SVI) for coagulation process was studied.
Results and discussion
RSM approach of optimization of COD and turbidity removal and SVI value
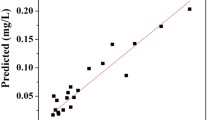
The statistical significance of the quadratic model was evaluated by the analysis of variance (ANOVA), as presented in Table 4. The empirical relationship between the COD removal (Y COD), turbidity removal (Y Tur) and SVI value (Y SVI) and the four test variables in coded units by the application of RSM is given by quadratic models, as given in Table 4. The adequacy of the model was tested through lack-of-fit, P values and F values (Vohra and Satyanarayana 2002). The P values for lack-of-fit were 0.4028, 0.6499 and 0.54488 for the removal of COD and turbidity and SVI value, respectively, which indicates that it is not statistically significant as is desirable. The insignificant value of lack-of-fit (>0.05) shows that the model is valid for the present study (Ziagova et al. 2007). The F values of 44.50, 54.61 and 156.90 were for COD removal and turbidity removal efficiency and SVI value, respectively, and P values were significant (P < 0.05) (Table 4). This means that the model terms are statistically significant and can predict response satisfactorily. The fit of the model was checked by determining the coefficient (R 2). In this study, the R 2 values obtained were 0.9765, 0.9880 and 0.9932 for the removal of COD and turbidity and SVI value, respectively. The closer the R 2 is to 1, the stronger is the model and the better it predicts the response. The value of the adjusted determination of the coefficient (adjusted R 2 = 0. 9545, 0.9628 and 0.9869) for COD and turbidity removal efficiency and SVI is also high, showing the high significance of the model. The values of the predicted R 2 at 0.8926, 0.9214 and 0.9708 obtained for the COD and turbidity removal efficiency and SVI value, respectively, were also high, supporting the considerable significance of the model. At the same time, the relatively low values of the coefficient of variation (CVCOD = 1.22, CVTur = 2.57 and CVSVI = 8.41) indicate a good precision and reliability of the experiments (Nakano and Jutan 1994; Collins et al. 2009). Adequate precision (AP) is a measure of the range in the predicted response relative to its associated error. Its desirable value is 4 or more. Adequate precision compares the range of the predicted values at the design points with the average prediction error. A ratio greater than 4 indicates an adequate model discrimination (Aghamohammadi et al. 2007; Ghafari et al. 2009). Usually it is necessary to check the fitted model to ensure that it provides an adequate approximation with the real system. Unless the model shows an adequate fit, proceeding with an investigation and optimization of the fitted response surface is likely to give poor or misleading results. Diagnostic plots such as the predicted versus actual values help us to judge the satisfactoriness of the model.
Figure 1 shows the plots for predicted versus actual values of the parameters for removal of the COD (a) and reduction of the turbidity (b) and SVI value (c). These plots indicate an adequate agreement between the uncoded data and the ones obtained from the models. By applying the diagnostic plots such as the ones for predicted versus actual values, the model adequacy can be judged. The regression equation after the ANOVA gave the level of COD and turbidity removal as a function of four factors, i.e., the dosages of alum and PACl, initial solution pH and initial COD. After applying multiple regression analysis on the experimental data, the results of the CCD design were fitted with a second-order full polynomial equation.
Effect of different alum and PACl dosage on COD and turbidity reduction
In coagulation processes, three factors, viz. kind of an inorganic coagulant, the coagulant dosage and the pH, play an important role in determining the coagulation process efficiency in wastewater. In treatments using inorganic coagulants, the optimum pH range in which metal hydroxide precipitates should be determined (Aguilar et al. 2002). Different coagulants affect different degrees of destabilization. The higher the valence of the counter ion, the more is its destabilizing effect and the less is the dose needed for coagulation (Birjandi et al. 2013). At high dose of metal ions (coagulant), a sufficient degree of oversaturation occurs to produce a rapid precipitation of a large quantity of metal hydroxide, enmeshing the colloidal particles which are termed as sweep floc (Hu et al. 2011).The effect of different levels of the alum and PACl doses on COD and turbidity removal efficiency can be predicted from the 3D plot, while the other two variables are at its middle level, as shown in Fig. 2. Figure 2a clearly shows that the COD removal efficiency increases with increasing coagulant dosages. The maximum COD removal (88.26 %) is shown with 657 mg/l dosage of alum and 1,500 mg/l dosage of PACl. Thus, for significant removal of COD, high doses of coagulants are needed. This is due to the presence of large amounts of organic matter in the wastewater and interaction with coagulants that can cause the suspended solids in the effluent to be oxidized, coagulated and then be settled as sludge; this process will reduce COD (Kumar et al. 2009). In Fig. 2b the effects of PACl dosage on turbidity reduction are not significant within the dosage range studied. The effect of increasing the PACl dosage only reveals a minor impact on the reduction-removal efficiency of turbidity. It can be seen that the maximum removal of turbidity (84.8 %) was obtained with approximately 853 mg/l dosage of alum and 53 mg/l dosage of PACl. The addition of PACl in the effluent and its mixing create proper coagulation condition and the flocs generated are denser than water, hastening the settling of the flocs. The PACl, having multivalent aluminum ions, neutralize the particle charges and the hydrolyzed aluminum flocs enmesh the colloids and drive to settle at high COD (Hu et al. 2011).
The use of alum alone is not a perfect method for treatment paper mill wastewater and it is best to investigate with the other methods treatment and coagulants that can be provide necessary standards for this effluent. Therefore, in paper-recycling wastewater treatment use of alum coupled with PACl could be the best results (Stephenson and Duff 1996).
Effect of solution pH and alum dose on COD and turbidity reduction
Changes in coagulants species and charge of the target compound can result due to variation in pH of the liquid media. In addition to governing the coagulant speciation, the media pH influences the extent of dissociation of the trace organic contaminants, and can result in compound-specific removal performance during application of a certain type of coagulant (Birjandi et al. 2013). The addition of metal coagulants depresses the wastewater pH to a lower value. The decrease in pH after the addition of coagulant may be due to the several hydrolytic reactions, which are taking place during coagulation, forming multivalent charged hydrous oxide species and generating H3O+ ion during each step, thus reducing the pH value (Kumar et al. 2011).
It has also been reported that the coagulant addition depresses pH to highly acidic levels, as the coagulant dose is highly correlated with pH (Chaudhari et al. 2007). It is supposed that improvement of flocculation pH may reduce the alum dose needs for the optimization of the process. Figure 3 presents the effect of different levels of the pH and the alum dose on the removal of COD and turbidity when other two variables at its middle level. Both the turbidity reduction and the COD removal efficiency increase with an decreasing pH adjustment till reach their highest value at the optimal pH, between which the reduction efficiency values start to decrease. This confirms the findings of (Ahmad et al. 2008). Figure 3a shows that the maximum COD removal (89.67 %) can be obtained at pH = 2.0, but Fig. 3b shows that the maximum turbidity reduction efficiency (79.73 %) occurs at pH = 7.82. It is well known that the pH of the solution affects the hydroxyl radical generating capacity (Zayas et al. 2007). According to Petala et al. (2006), at a lower pH and with a lower coagulant dosage, the only mechanism for destabilization of particles is through charge neutralization. At a low pH, because the aggregates are small in size, the mechanism of colloidal destabilization is mainly charge neutralization. It can be said that the highest range of pH exists between 6.0 and 8.0, beyond which the effluent quality deteriorates. The most important reasons for such behaviours are: 1) at low pH, the presence of monomeric aluminum species [Al3+, Al(OH)2+ and Al(OH)2 +] causes the anionic particles to neutral and deposition of pollutants settlement process is best performed due to the formation positive metal complexes that help to build flocs with the negative organic pollutants in wastewater, 2) with increase in pH, the concentration of dissolved aluminum is reduced by the formation of uncharged metal hydroxide [Al(OH)3] that leads to rapid precipation and 3) with further increase in pH, the species Al(OH)4 − is dominant which reduces coagulation effects and no coagulation occurs anymore (Ahmad et al. 2008).
Effect of initial COD and pH on final COD and turbidity removal efficiency
The experimental runs were conducted with five different initial COD values. The effect of different levels of the initial COD concentration and the pH on final COD and turbidity removal efficiency can be predicted from the 3D plot, while the other two variables are at its middle level, as shown in Fig. 4. Figure 4a shows that the maximum value of 89.67 % for COD removal is found with an initial COD of 940 mg/l and that the COD removal efficiency decreases with a high initial COD value and with COD of 250 mg/l a COD removal efficiency of 58 % is achieved. In contrast, the results obtained on turbidity show that high initial COD values result in high reduction efficiency (Fig. 4b). With COD of 250 mg/l (as the COD low limit value introduced to software), turbidity reduction efficiency of 42 % is achieved, while the maximum value of 95 % reduction is found at initial COD of 2,250 mg/l.
Sludge volume index (SVI)
The sludge produced in physical–chemical treatments is due to the organic matter and total solids in suspension that are removed and the compounds formed with the coagulants used, since practically all of the latter become part of the sludge solids. In general, the amount and characteristics of the sludge produced during the coagulation process depend on the coagulants used and on the operating conditions. To observe the volume and settling characteristics, the SVI was determined. The SVI in coagulation process is generally governed by three factors: high polymer effect, osmotic pressure effect and hydration effect (Wang et al. 2007). Figure 5a presents the effect of different levels of the alum and PACl dosages on the SVI value when other two variables at its middle level. From this figure, the coagulant dosages of alum coupled with PACl have a significant interaction on the SVI, as shown in Fig. 5a. The curvilinear profile obtained for SVI is in accordance to the quadratic model. The SVI decreases significantly with coagulant dosage and pH, but is affected mostly by the coagulant dosage. The best condition of the minimum SVI (140 ml/g) is observed with dosage of 1,140 mg/l alum and of 1,495 mg/l PACl. Under strong acid conditions (e.g. pH < 4.5) and low ranges of COD, the aluminum ions are mostly in the form of Al3+, which is highly effective for decreasing the osmotic pressure and hydration effects. Figure 5b presents the effect of different levels of the pH and the initial COD concentration on the SVI value when the other two variables are at its middle level. All of these factors might be responsible for the much lower SVI under acid conditions (Fig. 5b). These results, observed from the response surface plot, are in good agreement with the fitted model for SVI obtained earlier. Figure 5c presents the effect of different levels of the PACl dose and the initial COD concentration on the SVI value when other two variables at its middle level. From this figure, the results obtained on SVI show that high initial COD values result in high SVI value. With COD of 750 mg/l (as the COD low limit value introduced to software), SVI value of about 340 ml/g is achieved, while the maximum value of 375 ml/g is found at initial COD of 1,750 mg/l.
Optimization using the desirability function
In the numerical optimization, we choose the desirable goal for each factor and response from the menu. The possible goals are: maximize, minimize, target, within range, none (for responses only) and set to an exact value (factors only). A minimum and a maximum level must be provided for each parameter included. A weight can be assigned to each goal to adjust the shape of its particular desirability function. The goals are combined into an overall desirability function, an objective function that ranges from zero outside of the limits to one at the goal. The selected program in numerical optimization seeks to maximize this function. The goal seeking begins at a random starting point and proceeds up the steepest slope to a maximum. There may be two or more maximums because of curvatures in the response surface and their combination into the desirability function. By starting from several points in the design space, chances improve for finding the best local maximum. A multiple response method was applied for optimizing any combination for four goals, namely, the initial solution pH, alum dosage, PACl dosage and initial COD. The numerical optimization found a point that maximizes the desirability function. The importance of each goal was changed in relation to the other goals. Optimized conditions and results of experiments for final COD and turbidity removal efficiency and SVI value are given in Table 5. The best optimum value for final COD and turbidity removal efficiencies were 80.02 and 83.23 %, respectively, and desirability value was close to 1. The obtained values of desirability showed that the estimated function may represent the experimental model and desirable conditions. In Table 6, the results of this study are compared with the results of other researchers; although wastewater and coagulants differ, but the use of alum coupled with PACl lead to remove most pollutants from wastewater is corollary to our methods. In our previous published paper, the purpose was aimed to examine the efficiency of alum and PACl (coagulants) in combination with a cationic polyacrylamide (C-PAMs, i.e., chemfloc 1510c and chemfloc 3876 as flocculating agents) in the removal of COD and turbidity from paper-recycling wastewater. The results demonstrated that the maximum amounts of 40 mg/l coagulant dosage and 4.5 mg/l flocculant dosage at pH 4.5 were required to give 92 % removal of turbidity, 97 % removal of COD and 80 ml/g value of SVI. However, the best coagulant and flocculant were alum and chemfloc 3876 at a dose of 41 and 7.52 mg/l, respectively, at pH of 6.85. In these conditions, the highest removal of COD and turbidity and lowest value of SVI were found to be 91.30 and 95.82 % and 12 ml/g, respectively.
Conclusions
Coagulation process is one of the simple and common physical–chemical methods, advocated to be used for paper-recycling wastewater treatment. Although there are many types of coagulants available to treat water and wastewater, opting the most effective coagulant for a particular wastewater is important. The treatment of pulp and paper mill wastewater using alum coupled with PACl as coagulants enhanced the reduction removal of turbidity and COD and produced a lower volume of sludge compared to the results obtained when the coagulants were used alone. A desirable functional approach was used to obtain a compromise between three different responses, i.e., COD, turbidity removal and SVI. The optimum conditions obtained were with 1,550 mg/l alum coupled with 1,314 mg/l PACl at pH 9.5, with 80.02 % of COD removal, SVI of 140 ml/g and 83.23 % of turbidity removal. The results showed good agreement between the experimental and model predictions.
References
Afzal M, Shabir G, Hussain I, Khalid ZM (2008) Paper and board mill effluent treatment with the combined biological-coagulation-filtration pilot scale reactor. Bioresour Technol 99(15):7383–7387
Aghamohammadi N, HbA Aziz, Isa MH, Zinatizadeh AA (2007) Powdered activated carbon augmented activated sludge process for treatment of semi-aerobic landfill leachate using response surface methodology. Bioresour Technol 98(18):3570–3578
Aguilar MI, Sáez J, Lloréns M, Soler A, Ortuño JF (2002) Nutrient removal and sludge production in the coagulation-flocculation process. Water Res 36(11):2910–2919
Ahmad AL, Wong SS, Teng TT, Zuhairi A (2008) Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater. Chem Eng J 137(3):510–517
Anderson-Cook CM, Borror CM, Montgomery DC (2009) Response surface design evaluation and comparison. J Stat Plan Inference 139(2):629–641
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Birjandi N, Younesi H, Bahramifar N, Ghafari S, Zinatizadeh AA, Sethupathi S (2013) Optimization of coagulation-flocculation treatment on paper-recycling wastewater: application of response surface methodology. J Environ Sci Health, Part A 48(12):1573–1582
Chamarro E, Marco A, Esplugas S (2001) Use of fenton reagent to improve organic chemical biodegradability. Water Res 35(4):1047–1051
Chaudhari P, Mishra I, Chand S (2007) Treatment of biodigester effluent with energy recovery using various inorganic flocculant. Colloids Surf A Physicochem Eng Aspects 296:238–247
Collins LM, Dziak JJ, Li R (2009) Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods 14(3):202–224
Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163(2–3):650–656
Helble A, Schlayer W, Liechti P-A, Jenny R, Möbius CH (1999) Advanced effluent treatment in the pulp and paper industry with a combined process of ozonation and fixed bed biofilm reactors. Water Sci Technol 40(11–12):343–350
Hu XJ, Wang JS, Liu YG, Li X, Zeng GM, Bao ZL, Zeng XX, Chen AW, Long F (2011) Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J Hazard Mater 185 (1):306–314. doi:10.1016/j.jhazmat.2010.09.034
Kumar R, Singh R, Kumar N, Bishnoi K, Bishnoi NR (2009) Response surface methodology approach for optimization of biosorption process for removal of Cr(VI), Ni (II) and Zn (II) ions by immobilized bacterial biomass sp. Bacillus brevis. Chem Eng J 146 (3):401–407. doi:10.1016/j.cej.2008.06.020
Kumar P, Teng TT, Chand S, Wasewar KL (2011) Treatment of paper and pulp mill effluent by coagulation. Int J Civil Environ Eng 3(3):222–227
Latorre A, Malmqvist A, Lacorte S, Welander T, Barceló D (2007) Evaluation of the treatment efficiencies of paper mill whitewaters in terms of organic composition and toxicity. Environ Pollut 147(3):648–655
Nakano E, Jutan A (1994) Application of response surface methodology in controller fine-tuning. ISA Trans 33(4):353–366
Oguz E, Keskinler B (2008) Removal of colour and COD from synthetic textile wastewaters using O3, PAC, H2O2 and H2CO3. J Hazard Mater 151(2–3):753–760
Petala M, Tsiridis V, Samaras P, Zouboulis A, Sakellaropoulos GP (2006) Wastewater reclamation by advanced treatment of secondary effluents. Desalination 195(1–3):109–118
Rodrigues AC, Boroski M, Shimada NS, Garcia JC, Nozaki J, Hioka N (2008) Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis. J Photochem Photobiol A Chem 194(1):1–10
Stephenson RJ, Duff SJB (1996) Coagulation and precipitation of a mechanical pulping effluent–I. Removal of carbon, colour and turbidity. Water Res 30(4):781–792
Thompson G, Swain J, Kay M, Forster CF (2001) The treatment of pulp and paper mill effluent: a review. Bioresour Technol 77(3):275–286
Vohra A, Satyanarayana T (2002) Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochem 37(9):999–1004
Wang J-P, Chen Y-Z, Ge X-W, Yu H-Q (2007) Optimization of coagulation-flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloids Surf Physicochem Eng Aspects 302(1–3):204–210
Zayas T, Rómero V, Salgado L, Meraz M, Morales U (2007) Applicability of coagulation/flocculation and electrochemical processes to the purification of biologically treated vinasse effluent. Sep Purif Technol 57(2):270–276
Ziagova M, Dimitriadis G, Aslanidou D, Papaioannou X, Litopoulou Tzannetaki E, Liakopoulou-Kyriakides M (2007) Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol 98(15):2859–2865
Acknowledgments
The present research was made possible by the sponsorship and financial support of the Ministry of Science of Iran and the Tarbiat Modares University (TMU). The authors wish also to thank Mrs Haghdoust (Technical Assistant of Environmental Laboratory) of TMU for her cooperation and Ellen Vuosalo Tavakoli (University of Mazandaran) for the final editing of the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Birjandi, N., Younesi, H. & Bahramifar, N. Treatment of wastewater effluents from paper-recycling plants by coagulation process and optimization of treatment conditions with response surface methodology. Appl Water Sci 6, 339–348 (2016). https://doi.org/10.1007/s13201-014-0231-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0231-5