Abstract
In the current work, the commercially available ZnO photocatalyst was used to investigate the photodecoloration of Acid yellow 99 (AY99) dye under solar light radiation. Promising enhancement of photodecoloration of AY99 dye was also achieved by the addition of β-cyclodextrin (β-CD) with the ZnO (ZnO–β-CD). The effects of process parameters such as initial concentration, pH, catalyst loading, and illumination time on the extent of decoloration were investigated. The optimum catalyst loading was observed at 2.0 g/L. The higher photoactivity of ZnO–β-CD/solar light system than ZnO/solar light system can be ascribed due to the ligand to metal charge transfer (LMCT) from β-CD to ZnII. The complexation patterns have been confirmed with UV–visible and FT-IR spectroscopy and the interaction between ZnO and β-CD has been characterized by FE-SEM, powder XRD analysis, and UV–visible diffuse reflectance spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic dyestuffs are extensively used in textile, printing industries, paper, and dye houses due to their ease of production, variety of colors, and fastness compared to natural dyes. Many commercially available dyes are known and approximately 1 million tons of these dyes are produced annually worldwide. It has been estimated that more than 10 % of the total dyestuff used in dyeing processes is released into the environment (Habibi and Esfahani 2007). Therefore, they are neither readily degraded nor removed by conventional wastewater treatment. Further, their hydrophilicity limits their removal by coagulation/flocculation, which, in any case, produces large amounts of sludge with consequent disposal problems. However, since even low levels are clearly visible and exert a significant environmental impact, it is necessary to develop effective treatment methods (Habibi et al. 2007).
Various methods are available for the treatment of polluted water. Biological treatment is cost-effective and the majority of dyes are only adsorbed on the sludge and are not degraded. Physical methods such as ion exchange, adsorption, air stripping etc., are also ineffective and they simply transfer the pollutants to another phase rather than destroying them (Sauer et al. 2002). Photocatalysis is an alternative or complementary technology for the treatment of polluted water that has been widely reported (Byrne et al. 2002). The application of illuminated semiconductors for the decomposition of organic contaminants in water has been successfully used for a wide variety of compounds. Over the years, a large number of semiconductors have been used as photocatalysts. The most commonly studied photocatalysts are TiO2, ZnO, and CdS. ZnO appears to be a suitable alternative to TiO2 since its photodegradation mechanism has been proven to be similar to that of TiO2 (Rao et al. 2009). Recently, various reports have shown that ZnO is one of the potential photocatalyst materials. For instances, scientists found that ZnO nanoparticles exhibit higher rate than that of TiO2 nanoparticles (Velmurugan and Swaminathan 2011; Tian et al. 2012).
ZnO is a direct wide bandgap (3.37 eV) semiconductor, is deemed to be one of the most important semiconductor photocatalysts because of its high photosensitivity and stability (Li and Wang 2011). However, despite its great potential, the photocatalytic efficiency remains very low because of the fast recombination of the photogenerated electron–hole pairs in the single phase semiconductor (Hoffmann et al. 1995). Moreover, the wide bandgap semiconductors can only be excited by UV light, which occupies <10 % of the total energy of the solar radiation (Liao et al. 2006). Therefore, the development of visible-light-driven photocatalysts with high energy transfer efficiency has become one of the most challenging tasks in these days. Therefore, a number of efforts have been attracted to inhibit the recombination of electron–hole pairs and improve charge transport via coupling the wide band gap semiconductor photocatalysts with other materials (Li and Wang 2011), such as metal-doped ZnO composite (Kong et al. 2010; Mohan et al. 2012), polymer modified ZnO composite (Qiu et al. 2008), carbon nanotube (CNTs) (Saleh et al. 2011) or graphene—ZnO composites (Fu et al. 2012; Luo et al. 2012).
β-Cyclodextrin (β-CD) is an another material for enhancing the photocatalytic activity of semiconductor photocatalyst. CD-modified semiconductor nanocomposites have attracted renewed interest since Willner and colleagues observed that β-CD could stabilize TiO2 colloids and facilitate interfacial electron transfer processes (Willner and Eichen 1987). Cyclodextrins (CDs) are non-reducing cyclic maltooligosaccharides produced from starch by cyclodextrin glycosyltransferase and are composed of a hydrophilic outer surface and a hydrophobic inner cavity. CDs can form inclusion complexes with organic pollutants and organic pesticides to reduce the environmental impact of the chemical pollutants (Ishiwata and Kamiya 1999; Lu et al. 2004; Velusamy et al. 2014; Wang et al. 2006; Zhang et al. 2009, 2010, 2011, 2012, 2013). In this study, the activity of ZnO and the effect of addition of β-CD with ZnO on photocatalytic decoloration of Acid Yellow 99 (AY99) dye solutions under solar light radiation have been studied and the results are documented.
Materials and methods
Materials
The commercial organic dye AY99 (λmax = 445 nm) received from Loba Chemie was used as such. The semiconductor photocatalyst ZnO was purchased from Merck Chemicals. ZnO had a particle size 0.1–4 μm and surface area 5 m2/g. β-Cyclodextrin was received from Himedia chemicals. All other chemicals were of the Analytical grade received from Merck and used without further purification. Double distilled water was used to prepare all the experimental solutions. The physical properties and structures of AY99 dye and β-CD are shown in Table 1.
Characterization
FE-SEM was used to investigate the morphology of the samples β-CD, ZnO, and ZnO–β-CD. FE-SEM images were obtained on a Carl ZEISS (ΣIGMA Series, Germany) microscope taken at an accelerated voltage of 2 kV. X-ray diffraction patterns of powder samples were recorded with a high resolution powder X-ray diffractometer model RICH SIERT & Co with Cu as the X-ray source (λ = 1.5406 × 10−10 m). UV–Vis diffuse reflectance spectra were recorded on a Shimadzu 2550 UV–Vis diffuse reflectance spectrophotometer with BaSO4 as the background between 200 and 700 nm. UV–visible spectra were recorded by a UV–visible spectrophotometer (Shimadzu UV-1700) and the scan range was from 400 to 700 nm. FT-IR spectra were recorded using (Shimadzu 8400S) in the region 4,000–400 cm−1 using KBr pellets.
ZnO–β-CD preparation for characterisation
To study the interaction of β-CD on ZnO surface, a suspension containing 2.0 g/L ZnO and 10.0 g/L β-CD was magnetically stirred for 24 h and then centrifuged, later the solid phase was collected. After being centrifuged, the solid phase of the suspension was cautiously washed with double distilled water until no β-CD was detected in the supernatant liquid by phenolphthalein colorimetry (Zhang et al. 2012). Eventually, the ZnO–β-CD sample was dried at 50 °C. The sample prepared in this way was used for FE-SEM, XRD, and UV–DRS analysis. The calculated adsorption capacity of β-CD onto ZnO is 1.176 μmol/g as calculated by Langmuir isotherm model.
For studying the inclusion complex between β-CD and AY99 dye, equimolar ratio of solid AY99 dye and β-CD were dissolved in minimum amount of water was magnetically stirred for 24 h and then the complex was dried at 50 °C. This complex was characterized by FT-IR spectral analysis.
Photocatalytic decoloration experiment
In the photodecoloration of AY99 dye under solar light irradiation, sun light is used as an irradiation source. The intensity of sun light on the day of irradiation was measured using Lux meter. Exactly, 50 ml of the reaction solutions was taken in clean wide mouth glass vessels which were kept at a distance of 20 cm between each. The direct sun light radiation was allowed to fall on the reaction solutions during noon. The pH values of dye solutions were adjusted using digital pen pH meter (Hanna Instruments, Portugal) depending on desired values with HCl and NaOH solution as their effect on the adsorption surface properties of ZnO is negligible. The pH of the dye solutions was adjusted before irradiation process and it was not controlled during the course of the reaction. Prior to irradiation, ZnO suspensions were kept in dark for 10 min to attain adsorption–desorption equilibrium between dye and ZnO system. The reaction vessels were taken out at different intervals of time and the solutions were centrifuged. The supernatant liquid was collected and labeled for the determination of concentrations for the remained dye by measuring its absorbance (at λmax = 445 nm) with visible spectrophotometer (Elico, Model No. SL207). In all the cases, exactly 50 mL of reactant solution was irradiated with required amount of photocatalysts.
By keeping the concentrations of AY99 dye-β-CD as constant with the molar ratio of 1:1, the effects of the other experimental parameters on the rate of photocatalytic decoloration of AY99 dye solutions were investigated. The natural pH of AY99 dye solution was 5.4 and the irradiation time was fixed as 120 min.
Decoloration kinetics
The photocatalytic decoloration process of AY99 dye tends to follow pseudo-first order kinetics in the presence of catalysts used in this study. The regression curve of natural logarithm of AY99 dye concentration vs. illumination time shows a straight line using the formula,
where Co and Ct represent the initial concentration of the corresponding AY99 dye in solution and that of illumination time of t, respectively, and k represents the apparent rate constant (min−1) (Chun et al. 2001; Zertal et al. 2004; Rao et al. 2009).
Dissociation constant measurements
The dissociation constant (KD) value for the complexation between β-CD and AY99 dye can be calculated using the Benesi–Hildebrand equation (Velusamy et al. 1996).
where,
[C], [S] represent the concentrations of the β-CD, AY99 dye molecules, respectively, at equilibrium. ΔOD, the increase in absorption upon addition of β-CD; Δε, the difference in molar extinction coefficients between the bound and the free AY99 dye; KD, dissociation constant.
KD can be obtained from the ratio of the intercept (KD/Δε) and the slope (1/Δε) from the linear plot of [C] [S]/ΔOD versus {[C] + [S]}. The determined KD value is 4.8846 × 10−5 M.
Results and discussion
Field emission scanning electron microscopy
Figure 1 depicts FE-SEM micrograph of the bare β-CD, bare ZnO, and ZnO–β-CD, respectively. Bare β-CD shows amorphous surface. The surfaces of bare ZnO and ZnO–β-CD exhibit a similar morphology which indicates that the morphology of ZnO has not been affected by its complexation with β-CD. However, the external frontier of the ZnO–β-CD was noticeably different from ZnO. This may due to the aggregation of ZnO and β-CD particles as the surfaces of the particles are very loose. This kind of surface arrangement can provide a superior adsorption environment and more active site for the photocatalytic reaction.
X-ray powder diffraction analysis
The X-ray powder diffraction patterns of β-CD, 1:1 physical mixture ZnO–β-CD, and ZnO are presented in Fig. 2a–c, respectively. XRD pattern of the sample is summarized in Fig. 2. All the diffraction peaks of samples can be indexed as the wurtzite structured hexagonal ZnO with lattice (JCPDS, No. 36-1451), which indicated that the sample is pure ZnO. The high intensities of the XRD peaks of the sample suggest that the ZnO phase used in this work is highly crystalline. Diffraction peaks at 31.73°, 34.45°, 36.28°, 47.51°, and 56.68° correspond to (100), (002), (101), (102), and (110) planes of ZnO, respectively. Moreover, the addition of β-CD do not cause any shift in peak position of that of ZnO phase. The results also demonstrated that the ZnO conserved their wurtzite crystal features. Addition of β-CD causes no effect on the crystalline feature of ZnO.
UV–visible diffuse reflectance spectra
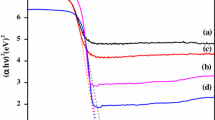
The diffuse reflectance spectra of ZnO and ZnO–β-CD catalysts are provided in Fig. 3, respectively. As shown in the Fig. 3b, ZnO–β-CD has slightly higher absorption intensity in the visible region compared to the bare ZnO (Fig. 3a), this is attributed to charge transfer ligand to metal charge transfer (LMCT) from β-CD to ZnII.
UV–visible and FT-IR spectral analysis
The molecular structure of β-CD allows various guest molecules with suitable dimensions to form host/guest inclusion complexes. In this study, the inclusion complex between β-CD and AY99 dye was characterized with UV–visible spectrum as given in Fig. 4. Figure 4 depicts that the absorbance of inclusion complex increases with increasing the concentration of β-CD (Wang et al. 2006).
Though IR measurements are not employed for detecting inclusion compounds (due to the superposition of host and guest bands), in some cases where the substrate has characterstic absorbance in the regions where β-CD does not absorb, IR spectrum is useful. From the FT-IR spectra (Fig. 5), it was observed that the peaks corresponding to monosubstituted benzene (1,596, 1,544 and 1,514 cm−1) for the AY99 dye molecule (Fig. 5b) are presented in the 1:1 physical mixture of β-CD-AY99 dye complex (Fig. 5c), whereas hidden in the β-CD-AY99 dye 1:1 complex (Fig. 5d). Moreover, it contains all the absorption peaks related to β-CD (Fig. 5a). It is interesting to note that the spectrum of a physical mixture of β-CD and AY99 dye resemble more of the AY99 dye peaks than that of a complex spectrum. It clearly confirms that there is an inclusion of the AY99 dye molecule inside the β-CD cavity in such a way that, the complexation between the AY99 dye molecule and β-CD has been proved by the FT-IR spectral data (Fig. 5).
Effect of initial dye concentration
The photocatalytic decoloration of AY99 dye was carried out at different initial concentrations ranging from 5.04 × 10−5 to 30.22 × 10−5 M in ZnO and ZnO–β-CD systems. The percentage removal of AY99 dye was decreased with increase in the concentration of dye. Nevertheless, the decoloration efficiency decreases because the photogeneration of holes or hydroxyl radicals on the catalyst surface is reduced (Guettai and Amar 2005). Under these conditions, the active sites of the catalyst are covered by dye molecules and degradation products, which compete with H2O and OH• to be adsorbed on the same sites, resulting in the lower formation of OH• radicals, the principal oxidant indispensable for high degradation efficiency (Sahel et al. 2007). In addition, the significant amount of light radiation that may be screened by dye molecules should be taken into account, since less photons reach the photocatalyst surface to further generate OH• (Guettai and Amar 2005; Camarillo and Rincon 2011).
Effect of initial pH
The solution pH appears to play an important role in the photocatalytic process of various pollutants (Krishnakumar et al. 2012). The effect of pH on the photodecoloration of AY99 dye was studied in the pH range 1–11. The Zero Point Charge (pzc) for ZnO is 9.0 ± 0.3. ZnO surface is positively charged below pH 9, whereas it is negatively charged above pH 9 based on their pzc (Bansal and Sud 2011). AY99 is an acidic dye in aqueous solution.
At acidic pH range, the removal efficiency is less and it is due to the dissolution of ZnO. Because ZnO is amphoteric in nature and is dissolved at lower pH, forming salts. At higher pH, it forms zincates such as [Zn(OH)4]2. In the basic pH, ZnO surface is negatively charged by means of adsorbed OH− ions. The presence of large quantities of OH− ions on the particle surfaces as well as in the reaction medium favors the formation of OH• (Sobana and Swaminathan 2007). All these factors are responsible for higher decoloration efficiency in basic pH.
Effect of amount of photocatalyst
The influence of the photocatalyst concentration from 0.5 to 3.0 g/L for AY99 dye on the photodecoloration efficiency was investigated. The observed results revealed that the photodecoloration efficiency increases with increase in ZnO concentration. This can be explained in terms of availability of active sites on the catalyst surface and the penetration of solar light into the suspension. The total active surface area increases with increasing catalyst dosage. Hence, the optimum amount of catalyst for photocatalytic decoloration of AY99 dye was found to be 2.0 g/L. All the process parameters were performed with this concentration of ZnO.
Effect of illumination time
Illumination time plays an important role in the decoloration process of the pollutants. The illumination time was varied from 30 to 180 min. The remaining concentration is decreased with an increase in the illumination time. It was observed that nearly 98.0 % decoloration of AY99 dye solution is achieved with in 180 min.
Figure 6 shows the maximum percentage removal of AY99 dye with various operational parameters. It is observed that ZnO–β-CD/Solar light system exhibits better photocatalytic decoloration efficiency than that of ZnO/Solar light system.
Mechanism of the effect of β-CD on photodecoloration
The proposed mechanism for the photocatalytic decoloration of AY99 dye by excitation of ZnO/β-CD is shown in Fig. 7. Since β-CD has higher affinity on ZnO surface than dye molecules, β-CD molecules could be adsorbed on ZnO surface and engage the active sites. β-CD would capture holes on active ZnO surface resulting in the formation of stable ZnO/β-CD inclusion complex. So, the inclusion complex reaction of β-CD with dye molecules should be the key step in photocatalytic decoloration in ZnO suspension containing β-CD. Dye molecules form inclusion complex resulting in the indirect photodecoloration to be the main reaction channel (Zhang et al. 2009). AY99 dye molecules enter into the cavity of β-CD, which is linked to the ZnO surface in the equilibrium stage and they absorb light radiation followed by excitation. An electron is rapidly injected from the excited dye to the conduction band of ZnO. Another important radical in illumination of ZnO–β-CD is the superoxide anion radical (•O2−). The dye and dye cation radical then undergo degradation.
β-CD/ZnO could show significant photocatalytic activity mainly because β-CD could trap the photo generated holes resulting in the lower e −CB /h +VB recombination (Zhang et al. 2010). In general, the lifetimes for the excited states of unreacted guests are prolonged when incorporated inside the cavity of CDs. Therefore, cyclodextrin facilitates the electron injection from the excited dyes to the ZnO conduction band and thereby enhances the decoloration (Zhang et al. 2011).
Conclusion
In this work, we carried out a detailed study of the effect of β-CD on the photodegradation of AY99 dye in ZnO suspension. XRD analysis exposes that ZnO conserved their wurtzite crystal features during the irradiation. Addition of β-CD does not cause any shift in peak position of that of ZnO phase. The results also demonstrated that the wurtzite ZnO conserved their wurtzite crystal features. UV–visible diffuse reflectance spectra exhibit that β-CD addition leads to a significant effect on the optical characteristics of ZnO. ZnO–β-CD system has faintly superior absorption intensity in the visible region compared to the bare ZnO system. Hence, photodecoloration of AY99 dye in ZnO–β-CD/solar light system exhibits better photocatalytic decoloration efficiency than that of ZnO/solar light system. This work provides essential information on the promotion effects of β-CD on the photodegradability of ZnO on dye in aqueous solution.
References
Bansal P, Sud D (2011) Photodegradation of commercial dye, Procion Blue HERD from real textile wastewater using nanocatalysts. Desalination 267(2–3):244–249
Byrne JA, Davidson A, Dunlop PSM, Eggins BR (2002) Water treatment using nano-crystalline TiO2 electrodes. J Photochem Photobiol A 148(1–3):365–374
Camarillo R, Rincon J (2011) Photocatalytic discoloration of dyes: relation between effect of operating parameters and dye structure. Chem Eng Technol 34(10):1675–1684
Chun H, Yizhong W, Hongxiao T (2001) Preparation and characterization of surface bond-conjugated TiO2/SiO2 and photocatalysis for azo dyes. Appl Catal B 30(3–4):277–285
Fu D, Han G, Chang Y, Dong J (2012) The synthesis and properties of ZnO–graphene nano hybrid for photodegradation of organic pollutant in water. Mater Chem Phys 132(2–3):673–681
Guettai N, Amar HA (2005) Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part I: parametric study. Desalination 185(1–3):427–437
Habibi MH, Esfahani MN (2007) Preparation, characterization and photocatalytic activity of a novel nanostructure composite film derived from nanopowder TiO2 and sol-gel process using organic dispersant. Dyes Pigment 75(3):714–722
Habibi MH, Esfahani MN, Egerton TA (2007) Preparation, characterization and photocatalytic activity of TiO2/methylcellulose nanocomposite films derived from nanopowder TiO2 and modified sol–gel titania. J Mater Sci 42(15):6027–6035
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95(1):69–96
Ishiwata S, Kamiya M (1999) Effects of humic acids on the inclusion complexation of cyclodextrins with organophosphorous pesticides. Chemosphere 38(10):2219–2226
Kong JZ, Li AD, Li XY, Zhai HF, Zhang WQ, Gong YP, Li H, Wu D (2010) Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J Solid State Chem 183(6):1359–1364
Krishnakumar B, Subash B, Swaminathan M (2012) AgBr–ZnO—an efficient nano-photocatalyst for the mineralization of Acid Black 1 with UV light. Sep Purif Technol 85:35–44
Li B, Wang Y (2011) Synthesis, microstructure, and photocatalysis of ZnO/CdS nano-heterostructure. J Phys Chem Solids 72(10):1165–1169
Liao SC, Lin HF, Hung SW, Hu CT (2006) dc thermal plasma synthesis and properties of zinc oxide nanorods. J Vac Sci Technol B 24(3):1322–1326
Lu P, Wu F, Deng N (2004) Enhancement of TiO2 photocatalytic redox ability by β-cyclodextrin in suspended solutions. Appl Catal B 53(2):87–93
Luo QP, Yu X, Lei BX, Chen HY, Kuang DB, Su CY (2012) Reduced graphene oxide hierarchical ZnO hollow sphere composites with enhanced photocurrent and photocatalytic activity. J Phys Chem C 116(14):8111–8117
Mohan R, Krishnamoorthy K, Kim SJ (2012) Enhanced photocatalytic activity of Cu-doped ZnO nanorods. Solid State Commun 152(5):375–380
Qiu R, Zhang D, Mo Y, Song L, Brewer E, Huang X, Xiong Y (2008) Photocatalytic activity of polymer-modified ZnO under visible light irradiation. J Hazard Mater 156(1–3):80–85
Rao AN, Sivasankar B, Sadasivam V (2009) Kinetic study on the photocatalytic degradation of salicylic acid using ZnO catalyst. J Hazard Mater 166(2–3):1357–1361
Sahel K, Perol N, Chermette H, Bordes C, Derriche Z, Guillard C (2007) Photocatalytic decolorization of Remazol Black 5 (RB5) and Procion Red MX-5B—Isotherm of adsorption, kinetic of decolorization and mineralization. Appl Catal B 77(1–2):100–109
Saleh TA, Gondal MA, Drmosh QA, Yamani ZH, AL-yamani A (2011) Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem Eng J 166(1):407–412
Sauer T, Neto GC, Jose HJ, Moreira RFPM (2002) Kinetics of photocatalytic degradation of reactive dyes in a TiO2 slurry reactor. J Photochem Photobiol A 149(1–3):147–154
Sobana N, Swaminathan M (2007) The effect of operational parameters on the photocatalytic degradation of acid red 18 by ZnO. Sep Purif Technol 56(1):101–107
Tian C, Zhang Q, Wu A, Jiang M, Liang Z, Jiang B, Fu H (2012) Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem Commun 48(23):2858–2860
Velmurugan R, Swaminathan M (2011) An efficient nanostructured ZnO for dye sensitized degradation of Reactive Red 120 dye under solar light. Sol Ener Mater Sol Cells 95(3):942–950
Velusamy P, Pitchumani K, Srinivasan C (1996) Selectivity in the bromination of aniline and N-substituted anilines encapsulated in β-cyclodextrin. Tetrahedron 52(10):3487–3496
Velusamy P, Pitchaimuthu S, Rajalakshmi S, Kannan N (2014) Modification of the photocatalytic activity of TiO2 by β-cyclodextrin in decoloration of ethyl violet dye. J Adv Res 5(1):19–25
Wang G, Wu F, Zhang X, Luo M, Deng N (2006) Enhanced TiO2 photocatalytic degradation of bisphenol A by β-cyclodextrin in suspended solutions. J Photochem Photobiol A 179(1–2):49–56
Willner I, Eichen Y (1987) TiO2 and CdS colloids stabilized by β-cyclodextrins: tailored semiconductor-receptor systems as a means to control interfacial electron-transfer processes. J Am Chem Soc 109:6863–6865
Zertal A, Gabor DM, Malouki MA, Sehili T, Boule P (2004) Photocatalytic transformation of4-chloro-2-methylphenoxyacetic acid (MCPA) on several kinds of TiO2. Appl Catal B 49(2):83–89
Zhang X, Wu F, Wang Z, Guo Y, Deng N (2009) Photocatalytic degradation of 4,4-biphenol in TiO2 suspension in the presence of cyclodextrins: a trinity integrated mechanism. J Mol Catal A 301(1–2):134–139
Zhang X, Wu F, Deng N (2010) Degradation of paracetamol in self assembly β-cyclodextrin/TiO2 suspension under visible irradiation. Catal Commun 11:422–425
Zhang X, Wu F, Deng N (2011) Efficient photodegradation of dyes using light-induced self assembly TiO2/β-cyclodextrin hybrid nanoparticles under visible light irradiation. J Hazard Mater 185:117–123
Zhang X, Li X, Deng N (2012) Enhanced and selective degradation of pollutants over cyclodextrin/TiO2 under visible light irradiation. Ind Eng Chem Res 51:704–709
Zhang X, Yang Z, Li X, Deng N, Qiana S (2013) β-Cyclodextrin’s orientation onto TiO2 and its paradoxical role in guest’s photodegradation. Chem Commun 49(8):825–827
Acknowledgments
The authors thank the Management and the Principal of Ayya Nadar Janaki Ammal College, Sivakasi, India for providing necessary facilities. Authors also thank the University Grants Commission, New Delhi, for the financial support through UGC-Major Research Project Ref. [UGC—Ref. No.F. No. 38-22/2009 (SR) Dated: 19.12.2009]. The instrumentation centre, Ayya Nadar Janaki Ammal College, Sivakasi and Department of Earthscience, Pondicherry University, Pondicherry are highly appreciated for recording the UV–visible, FT-IR spectra and Powder XRD patterns, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pitchaimuthu, S., Rajalakshmi, S., Kannan, N. et al. Enhancement of zinc oxide-mediated solar light decoloration of Acid Yellow 99 dye by addition of β-CD. Appl Water Sci 5, 201–208 (2015). https://doi.org/10.1007/s13201-014-0181-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0181-y