Abstract
Agricultural adsorbents are reported to have a remarkable performance for adsorption of dyes. In the present study, formaldehyde and sulphuric acid treated two agricultural adsorbents; potato peel and neem bark are used to adsorb methylene blue. On the whole, the acid-treated adsorbents are investigated to have high sorption efficiency compared to HCHO treated adsorbents. The percentage removal efficiency of H2SO4 treated potato peel (APP) increases considerably high from 75 to 100 % with increase in adsorbent dose, whereas the removal efficiency of H2SO4 treated neem bark (ANB) is found to be 98 % after adding the first dose only. The monolayer sorption behaviour of HCHO treated potato peel (PP) and APP is well defined by Langmuir, whereas the chemisorptions behaviour of HCHO treated neem bark (NB) and ANB is suggested by Temkin’s isotherm model. The maximum adsorption capacity measured is highest in ANB followed by NB, PP and APP with the values of 1000, 90, 47.62 and 40.0 mg/g, respectively. The pseudo-second-order kinetic model fitted well with the observed data of all the four adsorbents. The results obtained reveal that NB and ANB both are good adsorbents compared to PP and APP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demands of synthetic dyes have experienced significant growth in the past decades. Recent reports highlighted that more than 100,000 of various dyes are commercially available and annual worldwide production of dyestuffs surpassed to 7 × 105 metric tons (Pearce et al. 2003; Gong et al. 2005; Robinson et al. 2001). Extensive use of dyes and inefficiency in dying process leads to great loss of dyes to the main stream. The effluent from dying industry comprises moderate concentration (10–200 mg ml−1) of dyestuffs which contribute significant contamination of aquatic ecosystem (Neill et al. 1999). These stable compounds do not dissociate in environment and get accumulate in biota which further leave severe toxic and carcinogenic effects (Hu et al. 2006). Numerous physical, chemical and biological techniques are applied in past to attain maximum colour removal. Among them, adsorption technique is well accepted and considered to be an effective method for decreasing concentration of dissolved dyes in waste water (Reife et al. 1996). The adsorption process is fulfilled with little or no toxic pollutants and requires low initial capital and operating costs (Crini 2006).
Agricultural by-products have received significant interest in recent past by the researchers due to their abundance, non-hazardous nature and simple process of dye removal. Plentiful agricultural waste is used in recent past such as neem leaf powder (Bhattacharyya and Sharma 2004), tamarind fruit shell (Somasekhara Reddy 2006), jack fruit peel (Jayarajan et al. 2011), grapefruit peel (Abassi and Razzaghi 2009), rice husk (Abdelwahab et al. 2005), bark (McKay et al. 1999; Patil et al. 2011), sunflower seed husk (Ong et al. 2010) wheat straw (Hassanein Taha and Koumanova 2010) and saw dust (Shukla et al. 2002). These wastes have negligible economic value and often pose a disposal problem, so trying them as suitable adsorbent is the best alternative to utilize them.
In the present study, the removal efficiency of chemically treated potato peel and neem bark is observed on methylene blue (MB). The adsorption of MB by raw potato peel and neem bark has already been reported earlier as well by Öktem et al. 2012 and Srivastva and Rupainwar 2010, respectively. The novelty of the present research is to examine the efficiency of these adsorbents by modifying them with chemicals. One part of both the adsorbents is treated with HCHO, and other part is treated with H2SO4. The purpose of pre-treatment of raw adsorbent with formaldehyde is to remove the colour of raw adsorbents, which can interfere with the dye studied in order to achieve maximum colour removal with accuracy, whereas the acid treatment of both the adsorbents is done due to significant performance recorded by acid-treated adsorbents in past (Garg et al. 2003; Raghuvanshi et al. 2004; Parvathi et al. 2010). Adsorption isotherm and chemical kinetics are considered to find out the nature and mode of adsorption process. The present research will help in selecting the proficiently and economically suitable adsorbent for treatment of textile industry waste.
Materials and methods
Selection and preparation of sorbents
Two adsorbents potato peel and neem bark are selected as suitable adsorbents. Potato peel is obtained from mess of BMIET, while neem bark is collected from surrounding area of the same institute. Both the adsorbents are first washed with water and air dried in oven at 65 °C for 24 h. After drying, they are grinded and further treated with formaldehyde to restrict the integration of adsorbent’s own colour with the dye tint.
Formaldehyde treatment of raw adsorbents
For removing the colour of raw adsorbents, the grinded potato peel is dipped in 6 % HCHO overnight and washed further with distil water until the decanted water became colourless and pH reached at neutral. The colour-abated potato peel is then dried in oven at 70 °C and further sieved to obtain the particles of 80–150 μm mesh size. For removing the colour of neem bark, the grinded mixture is dipped in a solution of 37 % HCHO and 0.2 N H2SO4 overnight followed by soaking in NaHCO3 overnight to remove acid (Garg et al. 2003). After that, the same procedure of washing and drying is followed like potato peel. Finally the light brown coloured HCHO treated potato peel (PP) and dark brown coloured HCHO treated neem bark (NB) powders are obtained.
Acid treatment of the adsorbents
Both the adsorbents are treated with H2SO4 in 1:1 ratio and left overnight followed by washing with distil water many times until the pH reached at neutral. The black coloured powders of both the adsorbents are obtained after drying in oven at 105 °C and sieving through the mesh size of 80–150 μm mesh size.
The morphological and structural properties of all the four adsorbents are examined with the help of Hitachi, model S-3700, Scanning Electron Micrograph and Thermo Scientific Nicolet-380, FTIR.
Sorbet
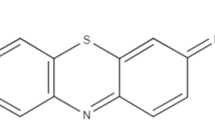
The dye, MB, chemical formula = C16H18ClN3S, MW = 319.86, λmax = 662 is selected as sorbet. The structure of dye is given below in Fig. 1.
Analytical procedure
Experiments are conducted by preparing 1,000 ppm of stock solution of MB. For determining the concentration of dye, Lambert–Beer’s method is used. Analysis is done on double-beam Chemito UV–Visible Spectrophotometer having the wavelength range of 190–1,100 nm and bandwidth of 1.6/1.0 nm. All the samples are examined on the recorded wavelength of 662 nm. Standard concentrations of MB (1–10 ppm) are prepared to obtain the calibration curve, and graph factor is calculated to get accurate results with minimum error. A comparative study of removal of dye on all the four adsorbents is studied by considering various parameters like adsorbent dose, concentration of dye, pH of the solution and time. The experiments are performed in batches at room temperature by shaking 50 ml of 100 ppm (except the concentration effects) dye solution with 0.250 g (except the dose effects) of each adsorbent taken in 100 ml of Erlenmeyer conical flask at the speed of 125 rpm.
Sorption of dye on various adsorbents is calculated by the mass balance equation given below where q denotes the amount of dye adsorbed on the adsorbent (mg/g); V denotes the volume of the solution (1); m denotes the amount of adsorbent taken in g; Ci and Cf denotes the dye concentration in mg/l before and after adsorption, respectively.
whereas percentage removal of dye is calculated by the given formula:
Experimental validation
Three different isotherm models, Langmuir, Freundlich and Temkin, are fitted to analyse the isotherm data. The initial concentrations for analysing the suitable isotherm model followed by all the four adsorbents are selected as 20, 50, 100, 150 and 250 ppm.
Langmuir’s and Freundlich’s equations are commonly used to express the isotherms at constant temperature for waste water treatment operations (Eren and Acar 2006). The linear form of Langmuir’s model supports the monolayer sorption hypothesis which occurs on the outer surface having infinite number of active sites. The empirical equation of Freundlich isotherm model is based on the sorption of dye over heterogeneous surface. Temkin’s isotherm model usually applied to observe the chemisorptions behaviour of sorbent and sorbet. The linear form of Langmuir’s, and empirical equation of Freundlich and Temkin’s isotherm models are, respectively, explained by the following expressions:
where in Langmuir’s equation, Ce is the equilibrium concentration (mg/l); qe is the amount of dye adsorbed (mg/g); Q0 is the maximum dye per gram of adsorbent (mg/g); and b is Langmuir’s constant related to energy of adsorption (l/mg). In Freundlich’s equation, KF is the constant related to sorption capacity. 1/n is an empirical parameter related to the sorption intensity of the adsorbent. A is Temkin’s constant representing sorbet–sorbent interactions, and B is another constant related to the heat of adsorption.
One more essential feature of Langmuir’s isotherm is determined which is a dimensionless constant separation factor (RL), expressed by the following equation given by Hall et al. (1966)
where C0 is initial concentration of dye (mg/l), and b is Langmuir’s constant (l/mg). The value of RL defines the nature of adsorption as below
RL > 1 = unfavourable, RL = 1 linear, RL = 0 irreversible, 0 < RL > 1 = favourable
The experimental data are analysed to observe the appropriate kinetic model followed by adsorption process including mass transfer and chemical reactions. Pseudo-first-order and pseudo-second-order kinetic models are used to describe the kinetics of adsorption. The integrated form of Lagergren first-order kinetic model is represented by the equation given below:
where k1 is the rate constant of first-order adsorption (1/min); qe is the amount of dye adsorbed in mg/g at equilibrium; and qt is the amount of dye adsorbed in mg/g at any time t. The linear form of pseudo-second-order kinetic model rate equation is expressed as:
where k2 is the rate constant of second-order adsorption (1/min) depends on the sorption capacity of solid phase. The initial sorption rate, h (mg/g min), is also calculated with the help of second-order kinetic model by the following expression:
Results and discussion
Morphological and structural study of adsorbents
All the four adsorbents obtained are analysed on SEM and FTIR to know about their morphological and structural properties. The SEM images of all the four adsorbents given in Fig. 2 show significant particle and pore size for the dye to be adsorb. From the images, it is evident that PP reflects the presence of smooth particles of size ranges up to 22–30 μm while NB shows enough rough shape particles of average size from 8.38 to 10.6 μm. The SEM images confirmed that both the adsorbents have sufficient pore size and surface area for the sorption of dye.
The comparative chart of FTIR spectra of all the four adsorbents represents clearly that almost all the adsorbents show more or less similar characteristics adsorption spectra. From the chart, it is well depicted that % transmittance is relatively greater in case of NB and H2SO4 treated neem bark (ANB) compared to PP and H2SO4 treated potato peel (APP). Table 1 elucidates various functional groups present on the surface of all the four adsorbents studied. These functional groups play major role in attracting the dye molecule from aqueous solution. It is well defined from the table that both potato peel and neem bark contain carboxylic group (hemicelluloses, pectin and lignin), phenolic (lignin, lipids and waxes), amines and sulphoxides group (Fig. 3).
Effect of dose
The effect of dose is studied by varying the amount of dose from 0.1 to 1.0 g of each adsorbent in 50 ml of 100 ppm of MB dye solution. Shaking is done at 125 rpm for 30 min at room temperature. It is clear from Fig. 4 that with increase in dose, the % removal of PP, NB and APP also increases from 75 to 93, 92 to 96 and 75 to 100 %, respectively. Maximum removal is achieved by APP. It may be due to presence of more active sites on the surface. Initial increase in adsorption is due to availability of more surface area on the adsorbents (Namasivayam et al. 1996; Garg et al. 2003), but further increase in dose saturates the adsorbents and steady state is observed. It is as a result of aggregation of adsorption sites by too much adsorbent which reduces the adsorption efficiency (Araujo and Teixeira 1997). The % removal of ANB decreases with increase in dose due to maximum absorbance (98 %) achieved with in short span of time, and further addition of adsorbent results in overcrowding which in turn decreases the efficiency. Unit adsorption of dye is decreased in all cases after increasing dose. This can be due to overlapping of adsorption sites as a result of overcrowding of adsorbent particles (Namasivayam et al. 1998).
Effect of concentration
Effect of dye concentration is studied by keeping adsorbent dose constant at 0.250 g in 50 ml of different concentration of dye varying from 20, 50, 60, 80, 100, 150 and 250. Shaking is done for 30 min at room temperature. Decolourization is observed in first 15 min at lower concentrations. The % removal of dye in case of PP, APP and NB decreases significantly from 85 to 63, 100 to 62 and 92 to 85 %, whereas in ANB, the % removal increases from 90 to 97 % as depicted by the Fig. 5. The decrease in removal can be attributed to saturation of sorption sites on the adsorbent as the concentration of dye increases (Tan et al. 2010), while initial increase in removal up to 100 mg/l and further saturation of ANB may be due to sufficient sorbent accessibility to adhere with the sorbet.
Effect of pH
Adsorption of dye on all the four adsorbents showed significant removal with change in pH. The optimum pH for removal of MB by PP, NB, APP and ANB is observed to be 8, 4, 12 and 2, respectively, as shown in Fig. 6. HCHO treated potato peel and APP showed significant variation in percent removal from 63 to 80 and 76 to 94 %, respectively, on changing pH from 2 to 14, while NB and ANB showed less variation in per cent removal from 93 to 83 and 98 to 90 %, respectively, by varying the same pH. HCHO treated neem bark and ANB showed good removal in acidic condition, while PP and APP showed significant removal in alkaline condition. Increase in negative charged ions on PP and APP favoured the adsorption of cationic dye in alkaline condition due to pectin present on surface of the two adsorbents (Flavio et al. 2008). Maximum removal of MB on NB and ANB in acidic medium may be due to the cellulosic compounds (carboxylic and phenolic groups) present on the adsorbent surface (Ferrero 2007).
Adsorption isotherm
The various isotherm (Langmuir, Freundlich and Temkin) parameters specified in Table 2 confirmed different type of sorption processes followed by the four adsorbents studied. From Table 2, it is clear that PP and APP responded good behaviour with all three types of isotherm models but Langmuir’s isotherm fitted quite well with them which is also well explained by the good regression coefficient of 0.99 and 0.96, respectively. The Langmuir’s model described the monolayer sorption nature of PP and APP. The maximum adsorption capacity of PP and APP obtained is 47.62 and 41.60 mg/g, respectively, which are higher than 33.55 mg/g measured by Öktem et al. 2012 on raw potato peel.
It is well defined from Table 2 that Temkin’s isotherm is best fitted with the adsorption behaviour of NB and ANB, showing good regression coefficient of 0.85 and 0.99, respectively, compared to other two isotherms studied. Temkin’s isotherm model reflects chemical adsorption mechanism between the dye and sorbent which takes into account the acquisition of the more energetic adsorption sites at first (Abdel-Ghani et al. 2007). The maximum adsorption capacity of neem bark powder for MB estimated by Srivastva and Rupainwar 2010 is 3.11 × 103 mol g−1(994 mg/g) which is higher than the NB efficiency of 90 mg/g but lower than the ANB efficiency of 1,000 mg/g measured.
In present study, the value of RL lies between 0.11 and 0.625 for PP and 0.03 and 0.31 for APP which are in the range of 0–1, indicating that the adsorption process is favourable for both the adsorbents. The value of n estimated is >1 for all the four adsorbents as depicted in Table 2. This represents that adsorption is favourable in nature.
Chemical kinetics
The kinetic plots of Eq. (8) and (9) are made at the initial concentration of 100 ppm for all the four adsorbents. From Table 3, it is concluded that the second-order kinetic model fitted well with the observed data of all the four adsorbents. Similar results are reported for sorption of MB on potato peel (Öktem et al. 2012) and neem bark (Srivastva and Rupainwar 2010). The initial adsorption rate h is higher in case of both NB and ANB as compared to PP and APP. The calculated values of qe for all the four adsorbents from second-order kinetic equation are found to be agreed well with the experimental values of qe.
Conclusion
The efficiency of four adsorbents PP, APP, NB and ANB for removing MB is evaluated by varying dose, pH and concentration. On varying dose, the % removal of PP, NB and APP also increases from 75 to 93, 92 to 96 and 75 to 100 %, respectively, due to availability of more surface area on the adsorbents, while the % removal of ANB decreases with increase in dose due to overcrowding of the adsorbent which in turn decreases the efficiency. On changing concentration, the % removal of dye in case of PP and APP decreases significantly from 85 to 63 and 100 to 62 % while little decrease is observed in case of NB and ANB from 92 to 85 and 90 to 97 %, respectively. The decrease in removal may be attributed due to saturation of sorption sites on the adsorbent as the concentration of dye increases (Tan et al. 2010). The optimum pH for removal of MB on PP, NB, APP and ANB are observed to be 8, 4, 12 and 2, respectively.
The maximum dye adsorption capacity, Q0 observed for NB and ANB, is 90.91 and 1,000 mg/g, respectively, which is >76.9 mg/g for the removal of MB on hazelnut shell as reported by Ferrero 2007. The Q0 estimated for NB in present study is lower than the value 3.11 × 103 mol/g estimated by Srivastva and Rupainwar 2010, but the later reported is less than the ANB sorption capacity of present study. The Q0 observed for PP and APP is 47.62 and 41.6 mg/g, respectively, which are higher than the efficiency measured for MB on cherry saw dust, Oak saw dust and pitch pine saw dust reported by Ferrero 2007.
The physical adsorption behaviour of PP and APP is well supported by Langmuir’s adsorption isotherm. HCHO treated neem bark and ANB followed chemisorptions nature which is acknowledged by the good correlation of Temkin’s isotherm. SEM and comparative chart of FTIR spectra of all the four adsorbents are in favour of the results obtained. The second-order kinetic model fitted well with the observed data of all the four adsorbents.
From the present research, it is estimated that NB and ANB are better adsorbent than PP and APP but neem bark performed efficiently in acid treated nature whereas potato peel worked better in HCHO treated form.
References
Abassi MN, Razzaghi Asl (2009) Removal of hazardous reactive blue 19 dye from aqueous solution by agricultural waste. J Iran Chem Res 2:221–230
Abdel-Ghani NT, Hefny M, El-Chaghaby GAF (2007) Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int J Environ Sci Technol 4(1):67–73
Abdelwahab O, Ahmed N, Amany ES, Khaled A (2005) Use of rice husk for adsorption of direct dyes from aqueous solution: a case study of Direct F. Scarlet. Egypt J Aquat Res 31(1):1110-0354
Araujo MM, Teixeira JA (1997) Trivalent chromium sorption on alginate beads. Int J Bio Deterior Biodegrad 40:63–74
Bhattacharyya KG, Sharma A (2004) Azadirachta indica leaf powder as an effective biosorbent for dyes: a case study with aqueous Congo red solutions. J Environ Manag 71:217–229
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Eren Z, Acar FN (2006) Adsorption of Reactive Black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination 194:1–10
Ferrero F (2007) Dye removal by low cost adsorbents: hazelnut shell in comparison with wood saw dust. J Hazard Mater 142:144–152
Flavio AP, Mazzocato AC, Gushikem Y (2008) Removal of methylene blue dye from aqueous solution by adsorption using yellow passion fruit peel as adsorbent. Bioresour Technol 99:3162–3165
Garg VK, Gupta R, Yadav AB, Kumar R (2003) Dye removal from aqueous solution by adsorption on treated saw dust. Bioresour Technol 89:121–124
Gong R, Li M, Yang C, Sun Y, Chen J (2005) Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J Hazard Mater B121:247–250
Hall KL, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid-diffusion kinetics in fixed bed adsorption under constant pattern conditions. Ind Eng Chem Fundam 5:212-223
Hassanein Taha F, Koumanova B (2010) Evaluation of adsorption potential of the agricultural waste, wheat straw for basic yellow 21. J Univ Chem Technol Metall 45(4):407–414
Hu ZG, Zhang J, Chan WL, Szeto YS (2006) The sorption of acid dye onto chitosan nano particles. Polymer 47:5838–5842
Jayarajan M, Arunachalam R, Annadura G (2011) Agricultural wastes of jackfruit peels nano-porous adsorbent for removal of rhodamine dye. Asian J Appl Sci 4(3):263–270
McKay G, Porter JF, Prasad GR (1999) The removal of dye colours from aqueous solutions by adsorption on low cost materials. Water Air Soil Pollut 114:423–438
Namasivayam C, Muniasamy N, Gayathri K, Rani M, Ranganathan K (1996) Removal of dyes from aqueous solutions by cellulose waste orange peel. Bioresour Technol 57:37–43
Namasivayam C, Prabha D, Kumutha M (1998) Removal of dyes by adsorption onto agricultural solid waste. Bioresour Technol 64:77–79
Neill CO’, Hawkes FR, Hawkes DL, Lourenco N, Pinheiro HM, Delee W (1999) Colour in the textile effluents-sources, measurements discharge consents and simulation: a review. J Chem Technol Biotechnol 74(11):1009–1018
Öktem YA, Soylu SGP, Aytan N (2012) The adsorption of methylene blue from aqueous solution by using waste potato peels; equilibrium and kinetic studies. J Sci Ind Res 71:817–821
Ong ST, Keng P, Lee SL, Leong MH, Hung YT (2010) Equilibrium studies for the removal of basic dye by sunflower seed husk (Helianthus annuus). Int J Phys Sci 5(8):1270–1276
Parvathi C, Maruthvanan T, Sivamani S, Prakash C, Koushik CV (2010) Role of tapioca peel activated carbon (TPAC) in decolourisation of red brown C4R reactive dye. Indian J Sci Tech 3(3):290–292
Patil S, Renukdas S, Patil N (2011) Removal of methylene blue, a basic dye from aqueous solution by adsorption using teak tree (Tectona grandis) bark powder. Int J Environ Sci 1(5):711–725
Pearce CL, Lloyd JL, Guthrie JT (2003) The removal of colour from textile waste water using whole bacterial cells: a review. Dye Pigment 58(3):179–196
Raghuvanshi SP, Singh R, Kaushik CP (2004) kinetics study of methylene blue dye Bioadsorption on bagasse. Appl Ecol Environ Res 2(2):35–43
Reife A, Freeman HS (1996) Environmental chemistry of dyes and pigments. Wiley, New York
Robinson T, Chandran B, Nigam P (2001) Studies on the production of enzymes by white-rot fungi for the decolourisation of textile dye. Enzym Microbiol Technol 29:575–579
Shukla A, Zhang YH, Dubey P, Margrave JL, Shukla SS (2002) The role of saw dust in removal of unwanted materials from water. J Hazard Mater B 95:137–152
Somasekhara Reddy MC (2006) Removal of direct dye from aqueous solution with an adsorbent made from tamarind fruit shell, an agricultural solid waste. J Sci Ind Res 65:443–446
Srivastva R, Rupainwar DC (2010) Liquid phase adsorption of indigo carmine and methylene blue on neem bark. Desalin Water Treat 24:74–84
Tan LS, Jain K, Rozaini CA (2010) Adsorption of textile dye from aqueous solution on pre-treated mangrove bark, an agricultural waste: equilibrium and kinetics studies. J Appl Sci Environ Sanit 5(3):283–294
Acknowledgments
The corresponding author extends sincere thanks to SSN College, D.U, and D.T.U. Delhi for performing the experimental work. The support of the staff of DCRUST, Murthal remained greatly admirable throughout the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Tiwari, D.P., Singh, S.K. & Sharma, N. Sorption of methylene blue on treated agricultural adsorbents: equilibrium and kinetic studies. Appl Water Sci 5, 81–88 (2015). https://doi.org/10.1007/s13201-014-0171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0171-0