Abstract
Layered double hydroxides (LDHs), also called anionic clays, consist of cationic brucite-like layers and exchangeable interlayer anions. These hydrotalcite-like compounds, with Zn and Al in the layers and chloride in the interlayer space, were prepared following the coprecipitation method at constant pH. The affinity of this material for Remazol Blue 19, RB19 [2-(3-(4-Amino-9,10-dihydro-3-sulpho-9,10-dioxoanthracen-4-yl) aminobenzenesulphonyl) vinyl) disodiumsulphate], was studied as a function of contact time, pH of the solutions LDH dose and the RB19/[Zn–Al–Cl] mass ratio. It was found that 48 h is enough time for the equilibrium state to be reached with maximum RB19 retention at pH of 9 for an LDH dose equal to 100 mg and with an RB19/[Zn–Al–Cl] mass ratio higher than 3. The adsorption isotherm, described by the Langmuir model, is of L-type. The results demonstrate that RB19 retention on LDHs occurs by adsorption on external surface when RB19/[Zn–Al–Cl] mass ratio is equal or <3 and by both adsorption and interlayer ion exchange for ratios higher than 3. A mechanism for removal of RB19 anion has been confirmed by X-ray diffraction, IR spectroscopy and TG analysis (TG and DTG curves).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industrial effluents from textile, tanneries or printing are polluting discharges that contain nonbiodegradable dyes (EL Gaini et al. 2008a). Their decontamination by conventional techniques seems ineffective in some cases.
The removal of colouring agents from textile effluents has attracted attention in the last few years, not only because of their toxicity, but also mainly due to their visibility. In recent years, many investigations have focused on several adsorbents. In the field of textile, special attention was paid to these industries by developing research to identify cost-effective methods of treatment of their releases coloured and meet required standards. A fair estimate of dye losses to the environment is about 1–2 % during their production and 1–10 % in their use (Forgacs et al. 2004). Improper disposal of dye-containing wastewaters that cause aesthetic problems concerns not only the scientists, but is now beginning also to draw the public authority’s attention and can generate toxic effects to aquatic life. Effluents from textile dyeing are constituted by complex mixtures of dyes, auxiliary chemicals, salts, acids, bases, organochlorines and sometimes heavy metals (Goncalves et al. 2000).
The removal of coloured contaminants is one of the main problems of treating this type of effluents, because of resistance to the biodegradability of dyeing, light, heat and oxidants (Sun and Yang 2003). Traditional treatments involving biological (Walker and Weatherley 2000) coagulation and electrochemical techniques (Zhou and He 2007; Beltran-Heredia et al. 2011) as well as membrane processes (Baouab et al. 2000) are generally ineffective for total colour removal. Wide range of other methods has been developed, like adsorption on organic and inorganic matrices, photocatalysis, chemical oxidation, microbiological or enzymatic decomposition (Hao et al. 2000).
The removal of synthetic dyes from wastewaters is especially difficult when reactive dyes are present, for which conventional wastewater treatment plants give low removal efficiency (Kumari and Abraham 2007).
Dyeing with reactive dyes, mostly applied to cellulosic fibres like cotton, consists in the formation of a covalent bond between the dye and the fibre, under alkaline pH conditions and high temperature. The hydrolysis of the dye also occurs as a secondary reaction leading to a low degree of fixation on the fibre and considerable losses of the hydrolyzed dye in the effluent.
Retention onto solid materials has been viewed as a procedure of choice (Crini 2006) for dye removal since it is an effective and economic physical method that could allow a complete decolourization of the wastewaters and their possible re-use.
Activated carbon is a widely used and effective adsorbent, but its use is limited by the high costs associated with its regeneration or replacement. Several studies have been reported on the preparation of activated carbons from solid wastes and their application to dye removal (Kadirvelu et al. 2003; Onal 2006). Low-cost materials, in their natural and modified forms, have been also extensively studied as alternative adsorbents for dyes (Ahmad et al. 2007; Gurses et al. 2006). Among the different adsorbents, layered double hydroxides (LDHs), also known as hydrotalcite-like compounds or anionic clays, are promising waste scavengers, particularly for dye molecules (Allmann 1968). Recent studies showed that the use of LDHs for the retention of organic anions has given very good results (De Roy et al. 1992; Lakraimi et al. 2000).
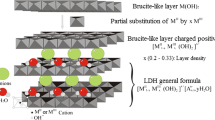
LDHs are solids which resemble the naturally occurring hydrotalcite. They can be described as positively charged layers of randomly distributed MII(OH)6 and MIII(OH)6 edge-sharing octahedra, similar to those in the brucite, Mg(OH)2, with the interlayer space being occupied by neutralizing hydrated Xm− anions. These materials can be represented by the general ideal formula:
where MII and MIII are divalent and trivalent metal ions, respectively; Xm− is the interlayer anion and x is defined as the MII/(MII + MIII) ratio.
The layered structure of LDHs, coupled with the high charge density of their sheets, their smooth and flexible structure, with relatively few interlayer bondings, are accountable for their important anion exchange and intercalation aptitudes allowing for the incorporation into their interlayer domains of a large number of inorganic and organic anions. The properties and applications of these compounds have been the subject of a number of general and specialized reviews (Rives 2001; Braterman et al. 2003; Zümreoglu-Karan and Ay 2012).
The aim of the present work is to assess the retention capacity of [Zn–Al–Cl] LDHs for the RB19 dye and to understand the mechanism involved. The influences of contact time, initial pH of the solutions, LDHs dose and the RB19/[Zn–Al–Cl] mass ratio have been investigated. The localization of the dye in the interlayer space and/or on external surfaces of the LDHs is studied by XRDIR and TG-DTG analyses.
Materials and methods
Adsorbent preparation and characterization
All experiments were carried out under a stream of N2 to avoid, or at least minimize contamination by atmospheric CO2. The [Zn–Al–Cl] LDH, with a [Zn]/[Al] ratio of 2, was synthesized by coprecipitation at a constant pH of 9.0 and at room temperature. Mixtures of molar ZnCl2 and AlCl3 aqueous solutions were slowly introduced into the reactor where the pH was maintained constant by the simultaneous addition of a 1.0-M NaOH solution. The resulting slurry was then stirred for 72 h at room temperature. The precipitate was filtered, washed several times with decarbonated water and then dried at room temperature (25 °C).
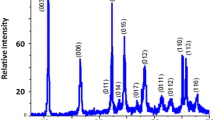
Characterization of the solid obtained by XRD and IR (Fig. 1) showed that the phase corresponds to a pure LDH (Miyata 1975). The solid consists of a well-crystallized single phase with large constituting crystallites. The lattice parameters refined on the hexagonal setting with a rhombohedral symmetry (space group: R-3 m) and the experimental metal ratios are given in Table 1.
Adsorbate
Remazol Blue 19, that was selected for this study, is a reactive textile dye. It was used in its commercial form. Table 2 presents its chemical and physical characteristics. In order to obtain hydrolysed RB19 aqueous solutions, the desired amount of the dye was dissolved in decarbonated water. The pH was adjusted by addition of NaOH and the volume was accurately made up.
Retention experiments
Retention experiments were carried out by the batch equilibrium technique at room temperature (25 °C), at constant pH, maintained by addition of NaOH, and under a stream of N2. Amounts of [Zn–Al–Cl] were dispersed in 100-mL RB19 solutions. The initial concentration of RB19 was varied between 15 and 100 mg/L. After filtration, the solid products obtained were dried at room temperature before being analyzed by XRD and IR techniques. The supernatants were recovered and the residual dye concentration was determined by UV–Vis spectroscopy. The absorbance was measured at 594 nm on a Spectronic Genesys 20 spectrophotometer.
The quantity of RB19 retained by [Zn–Al–Cl], Q, was calculated as the difference between initial and equilibrium (final) concentrations of the dye in solution (Ci and Ce, respectively) by mass of the adsorbent (m) in the volume of solution, V:
Analytical techniques
The XRD equipment used was a Siemens D 501 diffractometer. Samples of unoriented powder were exposed to copper Kα radiation (λ = 0.15415 nm). Measurement conditions were 2θ range 2–70°, step size: 0.08° 2θ, and step counting time: 4 s. Data acquisition was effective on a DACO-MP microcomputer. Unit cell constants were calculated using a least squares refinement.
Absorbance IR spectra were recorded on a Perkin-Elmer 16 PC spectrophotometer, at a resolution of 2 cm−1 and averaging over 100 scans, in the range 400–4,000 cm−1. Samples were pressed into KBr discs.
TG and DTG studies were performed in air with a SETARAM TG-DSC 92 instrument. Curves were recorded on 20 mg of the sample over a temperature range of up to 1,000 °C at a heating rate of 5 °C/min. The weights were corrected for the small effect of the gas flow as function of temperature.
Results and discussion
Preliminary adsorption experiments were conducted to determine the optimal conditions for the retention of RB19 on LDHs regarding the pH value, contact time (tc), initial concentration (Ci) of adsorbate and the mass ratio adsorbate /adsorbent.
Effect of pH
Generally pH is considered to be an important parameter that controls the retention at water-adsorbent interfaces (mLDH = 100 mg, Ci = 800 mg/L and tc = 48 h). The retention of RB19 by [Zn–Al–Cl] was studied at different pH values ranging from 4.5 to 10 (Fig. 2). It is shown that the RB19 retention is at its maximum in the pH range 7–8, which corresponds to the pH of natural water. At lower pH values (<7), the low retention observed may be attributed to a partial dissolution of the basic mineral matrix by acidic hydrolysis, which becomes more pronounced when the pH decreases. The low adsorption observed at pH range 9–10 may be explained by a competition with the carbonate ions for which the LDH is known to have a great affinity (Miyata 1975; Rey et al. 1992). The calibration of the solutions (LDH + RB19) at relatively basic pH was carried out in the absence of nitrogen and therefore is susceptible to be contaminated by atmospheric CO2. This hypothesis has been reported in other works (Legrouri et al. 2005; Lakraimi et al. 2006; EL Gaini et al. 2008b).
This phenomenon takes place despite the precautions taken during the preparation of the solid sample and the kinetics study, when the pH value is high. Following these experiments, it was decided to carry out the retention experiments at pH 7.
Effect of contact time (kinetic study)
The amount of RB19 retained by [Zn–Al–Cl] as a function of contact time, using a constant adsorbent mass of 100 mg and initial concentrations varied from 100 to 800 mg/L, is shown in Fig. 3. The kinetic study shows that the retention equilibrium state is reached after a contact time of 48 h since no change in the retained amount is detected afterwards. To be sure that the equilibrium state is reached for higher concentration, a RB19-LDHs contact time of 48 h was applied in the retention experiments.
A similar behaviour was obtained for other adsorbate with LDHs materials (EL Gaini et al. 2008a; Legrouri et al. 2005).
Adsorption isotherms
Figure 4a displays the retention isotherms of RB19 onto 40, 60 and 100 mg of LDH. The dye adsorption isotherms on [Zn–Al–Cl] can be considered clearly as pure L-type, indicating that the interaction sorbate–sorbent is much stronger than solvent–sorbent at the adsorption sites. Isotherms with this profile are typical of systems where the functional adsorbate is strongly attracted by the adsorbent, mostly by ion–ion interaction, which tends to reach a saturation value given by a nearly isotherm plateau. These results again suggest that RB19 anions are preferentially removed.
As may be seen, the RB19 retention is inversely proportional to the mass of LDH. The Qm value increases when the LDH mass decreases; this is somehow normal if we know that retention of RB19 by the LDH can be done, in addition to adsorption, also by intercalation between the layers. This intercalation requires an increasing molar ratio (RB19/Cl) and therefore relatively small mass of LDH to allow maximum exchange of the chloride ions by the dye’s ions.
The data for the uptake of RB19 have been processed in accordance with the linear form of Langmuir isotherm equation:
Q is the quantity of RB19 retained by the unit mass of LDH (g/g); Qm, the maximum quantity of RB19 retained by the unit mass of LDH (g/g); Ce, the equilibrium concentration of RB19 (mg/L) and K is the affinity constant of RB19 for LDH (L/g).
A linear relationship was observed among the plotted parameters (Fig. 4b), which indicates the applicability of the Langmuir’s equation. The sorption parameters obtained are summarized in Table 3.
These isotherms had an appearance reminiscent of those of the Langmuir adsorption isotherms. They provide a satisfactory linearization of retained amount, Q. This linearization was used to standardize the method of determining the maximum quantity Qm. It also provides the affinity constant, K, on which it is difficult to pronounce. In that order of size, the K values are at least comparable, suggesting that whatever the LDH mass used, the type of interaction between adsorbent and adsorbate is the same. However, the maximum adsorbed amount decreases when the mass of LDH is increased.
The XRD of the solids obtained by ion exchange of chloride by RB19 correspond to that of a hydrotalcite-type material. Figure 5 shows the XRD patterns for [Zn–Al–RB19] LDHs prepared with different anion concentrations. The layered structure of the material is preserved upon intercalation. However, the crystallinity is lowered, as attested by the broadening of the lines and the decrease in their intensity. In addition, intercalation is observed to cause a displacement of the (003) line, denoting an increase in the interlayer space which is due to the exchange of the chloride by the larger RB19 ions (Rubino 2001).
Furthermore, the adsorption on the surface does not affect this distance for low concentrations of the dye. By contrast, for relatively high concentrations, increased interlayer distance means that there is an exchange between chloride ions and the anions of RB19, which are larger and accompanied by the maximum occupancy of the available anionic sites.
The cell parameters for the best compound, in terms of crystallinity, were a = 0.306 nm, c = 2.775 nm and the interlayer distance d = 0.925 nm. The study of the effect of RB19 concentration indicates the existence of two phases, in terms of crystallinity, for 15 g/L (Fig. 5). The presence of the line at d = 0.778 nm indicates that the exchange is only partial in this case. In addition, the interlayer distance varies slightly with the ion concentration.
The IR spectra of the phases prepared (Fig. 6) are characteristic of LDH phases with the presence of bands reflecting the intercalation of RB19. The broad adsorption band around 3,400 cm−1 is due to the stretching of O–H groups of the hydroxide layer and interlayer water. In the low-frequency region, the bands at 809, 601 and 451 cm−1 are ascribed to the lattice vibration modes attributed to M–O and M–O–M vibrations (Legrouri et al. 2005). The asymmetric stretching vibration νa (S=O) appears in the region 1,150–1,260 cm−1. The absorption bands at 1,620 and 1,404 cm−1 are assigned to the stretching vibration of C=C in an aromatic ring, while the bands at 1,640, 1,300 and 1,188 cm−1 are assigned to those of C=O, C–O and C–N, respectively (Rubino 2001; Pouchert 1975; Pavia et al. 1996).
Effect of mass ratio RB19/LDH
The observation shows that the maximum adsorbed amount decreases when the mass of LDH is increased, which may seem strange but can be considered as normal with regard to the ion-exchange ability of the LDH. In fact, the ion exchange was observed to be requiring a high mass ratio (mRB19/mLDH > 3) and consequently a relatively low mass of the LDH allows for better ion exchange. It seems that this value corresponds to the optimum concentration of RB19 that is required to displace the chloride ions from within the LDH interlayer space. For mass ratios RB19/[Zn–Al–Cl] ≤3 (Fig. 7), the interlayer distance remains equal to that of the initial matrix which means that retention is only done by adsorption to the surface. In contrast, when this ratio is >3; we can see, on the diffraction patterns of the obtained products, another line at the low 2θ values. This line corresponds to a distance of 0.925 nm which proves the presence of a second phase intercalated by RB19 ions, that are larger than chloride (d = 0.778 nm). This means that the increase in the concentration of RB19 and therefore the mass ratio (RB19/LDH) facilitates the exchange reactions with a large amount of chloride ions by the anions of dye. Under these conditions, we can conclude that the elimination of the dye occurred according to two different processes:
-
RB19/[Zn–Al–Cl] ≤3: retention on the surface (adsorption).
-
RB19/[Zn–Al–Cl] >3: both adsorption and intercalation between the layers.
The retention of RB19 by the LDH attained 98 % for an RB19/LDH mass ratio of 15. This value compares well with the removal of RB19 by coconut coir activated carbon that attained 96 % (Chaudhuri et al. 2009).
TG and DTG analysis
TG and DTG curves obtained for [Zn–Al–RB19] phase are reported in Fig. 8b. The thermal evolution under air takes place in five consecutive stages with weight losses for which the inflexion points coincide with temperatures corresponding to minima and maxima in the DTG trace. The first weight loss which begins as soon as heating is applied with a first effect at 85 °C is attributed to the loss of adsorbed water molecules.
The second weight loss at 220 °C is assigned to the interlayer water. The third weight loss around 320 °C is attributed to the dehydroxylation of the brucite-like layers. This destruction of the hydroxylated sheet is held at the same temperature as the starting matrix [Zn–Al–Cl] (Fig. 8a). These three first stages are similar to those previously reported for hydrotalcite-like materials (Cavani et al. 1991; Labajos and Rives 1996). The fourth weight loss corresponds to the departure of chloride ions for the [Zn–Al–Cl] phase in the form of gaseous HCl, which results in a signal on the DTG curve centred at 630 °C. This behaviour is identical to that of the host matrix. This confirms that the exchange of chloride ions by the RB19 is partial.
The fifth weight loss centred at about 950 °C starting from 750 °C is due to the decomposition of RB19 anion by combustion reactions, probably in the form of H2O, CO2, NO2 and SO2. The combustion of the organic anion at higher temperatures may be probably due to slow kinetics of decomposition of the sulphonate groups and may confirm the hypothesis of a slow diffusion of SO2 in the oxide matrix already formed at such temperatures (Lakraimi et al. 2006).
Anyway, this TG study confirms the retention of RB19 by [Zn–Al–Cl] and confirms the results obtained by other characterization techniques.
Conclusion
The present study shows that the [Zn–Al–Cl] can be used as an effective adsorbent for the removal of the RB19 dye from aqueous solutions. The LDH was able to remove up 98 % of RB19 from solutions whose initial concentration was varied between 15 and 100 mg/L. The quantity eliminated was found to depend on LDH dose, RB19/LDH mass ratio, contact time and the pH of the dye solution. The adsorption is described by Langmuir-type isotherm due to the surface homogeneity. The results of three characterization techniques, XRD, IR and TG-DTG, agree and indicate that the retention phenomenon may have taken place following two ways: adsorption and anion exchanges when the RB19/LDH mass ratio is higher than 3 and adsorption only when the ratio is low. The intercalation of the organic ion in the layered host structure was clearly evidenced by the net increase in the basal spacing from 0.778 nm for [Zn–Al–Cl] to 0.925 nm in the new phase, [Zn–Al–RB19].
References
Ahmad AA, Hameed BH, Aziz N (2007) Adsorption of direct dye on palm ash: kinetic and equilibrium modeling. J Hazard Mater 141(1):70–76
Allmann R (1968) The crystal structure of pyroaurite. Acta Crystallogr B24:972–977
Baouab MHV, Gauthier R, Gauthier H, Chabert B, El Bakar Rammah M (2000) Immobilization of residual dyes onto ion-exchanger cellulosic materials. J Appl Polym Sci 77:171–183
Beltran-Heredia J, Sanchez-Martin J, Rodriguez-Sanchez MT (2011) Textile wastewater purification through natural coagulants. Appl Water Sci 1:25–33
Braterman PS, Xu ZP, Yarberry F (2003) Chemistry of layered double hydroxides. In: Auerbach SM, Carrado KA, Dutta PK (eds) Handbook of layered materials. Marcel Dekker, New York
Cavani F, Trifino F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11(2):173–301
Chaudhuri M, Elmolla ES, Othman RBt (2009) Removal of reactive dyes from aqueous solution by adsorption on coconut coir activated carbon. In: Second international conference on engineering technology (ICET 2009). 8–10 December 2009, Kuala Lumpur
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97(9):1061–1085
De Roy A, Forano C, El Malki K, Besse JP (1992) Anionic clays: trens in pillaring chemistry. In: Occeli ML, Robson HE (eds) Synthesis of microporous materials. Van Nostrand Reinhold, New York, 2:108–169
EL Gaini L, Lakraimi M, Sebbar E, Bakasse M (2008a) Removal of the methyl orange dye from water to zin-aluminium-chloride layered double hydroxides. J Optoelectron Adv Mater 10(6):1415–1420
EL Gaini L, Sebbar E, Boughaleb Y, Bakasse M, Lakraimi M, Meghea M (2008b) Removal of the herbicide 2,4-dichlorophenoxyacetate from water to calcined layered double hydroxides. Nonlinear Opt Quantum Opt 00:1–13
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971
Goncalves IMC, Gomes A, Bras R, Ferra MIA, Amorim MTP, Porter RS (2000) Biological treatment of effluent containing textile dyes. J Soc Dyers Colour 116:393–397
Gurses A, Dogar C, Yalçin M, Açikyildiz M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131(1–3):217–228
Hao OJ, Kim H, Chiang PC (2000) Decolorization of wastewater. Crit Rev Environ Sci Technol 30(4):449–505
Kadirvelu K, Kavipriya M, Karthika C, Radhika M, Vennilamani N, Pattabhi S (2003) Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour Technol 87(1):129–132
Kumari K, Abraham TE (2007) Biosorption of anionic textile dyes by nonviable biomass of fungi and yeast. Bioresour Technol 98(9):1704–1710
Labajos FM, Rives V (1996) Thermal evolution of Cr(III) ions in hydrotalcite-like compounds. Inorg Chem 35:5313–5318
Lakraimi M, Legrouri A, Barroug A, De Roy A, Besse JP (2000) Preparation of a new stable hybrid material by chloride–2,4-dichlorophenoxyacetate ion exchange into the zinc–aluminium–chloride layered double hydroxide. J Mater Chem 10:1007–1011
Lakraimi M, Legrouri A, Barroug A, De Roy A, Besse JP (2006) Synthesis and characterization of a new stable organo-mineral hybrid nanomaterial: 4-chlorobenzenesulfonate in the zinc-aluminium layered double hydroxide. Mater Res Bull 41:1763–1774
Legrouri A, Lakraimi M, Barroug A, De Roy A, Besse JP (2005) Removal of herbicide 2,4-dichlorophenoxyacetate from water to zinc-aluminium-chloride layered double hydroxides. Water Res 39:3441–3448
Miyata S (1975) The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties: I. The systems Mg2+–Al3+–NO3−, Mg2+–Al3+–Cl−, Mg2+–Al3+–ClO4−, Ni2+–Al3+–Cl− and Zn2+–Al3+–Cl−. Clays Clay Miner 23:369–381
Onal Y (2006) Kinetics of adsorption of dyes from aqueous solution using activated carbon prepared from waste apricot. J Hazard Mater 137:1719–1728
Pavia DL, Lampman GM, Kriz GS (1996) Introduction to spectroscopy. Harcourt Brace College Publisher, Orlando
Pouchert CJ (1975) The aldrich library of infrared spectra, 2nd edn. Aldrich Chemical Co, Milwaukee
Rey F, Fornés V, Rojo JM (1992) Thermal decomposition of hydrotalcites. An infrared and nuclear magnetic resonance spectroscopic study. J Chem Soc, Faraday Trans 88:2233–2238
Rives V (2001) Layered double hydroxides: present and future. Nova Science, New York
Rubino FM (2001) Separation methods for methotrexate, its structural analogues and metabolites. J Chromatogr B Biomed Sci Appl 764(1–2):217–254
Sun QY, Yang LZ (2003) The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res 37(7):1535–1544
Walker GM, Weatherley LR (2000) Biodegradation and biosorption of acid anthraquinone dye. Environ Pollut 108:219–223
Zhou M, He J (2007) Degradation of azo dye by three clean advanced oxidation processes: wet oxidation, electrochemical oxidation and wet electrochemical oxidation-A comparative study. Electrochim Acta 53:1902–1910
Zümreoglu-Karan B, Ay AN (2012) Layered double hydroxides—multifunctional materials. Chem Pap 66(1):1–10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Elkhattabi, E.H., Lakraimi, M., Badreddine, M. et al. Removal of Remazol Blue 19 from wastewater by zinc–aluminium–chloride-layered double hydroxides. Appl Water Sci 3, 431–438 (2013). https://doi.org/10.1007/s13201-013-0092-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0092-3