Abstract
Microalga, Isochrysis galbana, biomass was entrapped into alginate gel by liquid curing method in the presence of Ca(II) ions. The biosorption of chromium(III) by the entrapped live algal biomass was studied in a batch system. The effect of initial cadmium concentration, pH, temperature and liquid and solid ratio on Cr(III) removal was investigated. The maximum experimental biosorption capacities for entrapped live algal biomass were found to be 335.27 mg Cr(III) g−1 of dry algal biomass. The kinetics of chromium biosorption was slow; approximately 75 % of biosorption took place in 2 h. The percent adsorption increased with increase in pH; pH 5 of the solution was found to favor adsorption very strongly. The equilibrium biosorption data were evaluated by Langmuir and Freundlich isotherm models, and was best described by Langmuir and Freundlich isotherms. The biosorbent was characterized and evaluated, the functional groups –OH, –COOH and C=O were involved in the biosorption process. Since binding capacity was relatively high for immobilized live algal biomass, those algal forms are to be considered as suitable biosorbent for the removal of chromium in wastewater treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mining and metallurgical wastewaters are considered to be the major sources of heavy metal contamination, and economic and cost-effective methods for the removal of metals are needed. Over the years, a number of physical and chemical options, such as conventional methods including chromatography, have been contemplated to remediate the effluents. These methods are highly expensive when the contaminant concentrations are in the range of 10–100 mg L−1 (Patterson 1977). Using microorganisms as adsorbents for removal of heavy metal offers a potential alternative to existing methods. Biosorption, as it has been perceived thus far, could be considered for its economic edges as a possible alternative technique for metal removal/recovery. Compared with the free biomass, immobilized microorganisms are considered more suitable for industrial application due to better mechanical strength, chemical stability, physical morphology, antidegradation ability and, more importantly, repeated adsorption–desorption characteristics (Tsezos et al. 1989; Lloyd et al. 1997). Natural polymers such as alginate, chitosan, chitin and cellulose derivatives have been mostly used as the matrix for the immobilization of microbial cells via an entrapment technique (Kumar et al. 2009). A major advantage of alginate gel entrapment is that immobilized cells do not suffer extreme physical–chemical condition changes during the immobilization process. Permeability, null toxicity and transparency of formed matrix imply a very gentle environment for immobilized cells (Smidsrød and Skjåk -Braek 1990; Araújo and Andrade Santana 1996). Application of microalgal biomass to remove heavy metals from industrial wastewater is economically valuable, technically sound, socially acceptable and is attractive for industry.
Many algal species such as Heterosigma akashiwo (Hada) (Hideshi et al. 2000), Spirogyra species (Gupta et al. 2001a), C. vulgaris (Aksu 2001) and Chlamydomonas reinhardtii (Gülay et al. 2006) have been extensively studied for heavy metal biosorption, and the process mechanisms seem to be different from species to species. The –COOH, –NH2, –OH and –SH groups on microbial cell wall provide the binding sites for interaction of metal ions (Kuyucak and Volesky 1998). Chromium has a high hardness value and +2, +3 and +6 oxidation states. Chromium and its compounds are widely used in many industrial fields such as plating, alloying, dyeing, tanning, finishing, wood preserving and refractory technologies. Cr2+ is unstable state; while Cr3+ is bioelement forms strong complexes with oxygen (hard acid), but complicated and less toxic than Cr6+ (Gürü et al. 2008). Water containing a high concentration of Cr can cause serious environmental problems, as well as induce toxic and carcinogenic health effects in humans (Wu et al. 2008; Gupta et al. 2001b; Gupta and Ali 2004; Gupta and Rastogi 2008; Gupta et al. 2009).
The drinking water guideline level recommended by the Environmental Protection Agency (EPA) in the USA is 100 μg L−1 (Lazaridis and Asouhidou, 2003). The legal discharge limit of Cr(III) varies from 0.5 mg L−1 (in surface water) to 2.0 mg L−1 (in sewers), depending on the processing country and wastewater treatment methods (Mohan et al. 2006). Since the presence of chromium in water has led to an increasing awareness and concern of its detrimental effects to nature and human beings, chromium ions must be removed from wastewater. This study was aimed at investigating the role of the functional groups present in the biomass in the biosorption process by FTIR analysis, in addition to the environmental parameters affecting the biosorption process such as pH, time, initial metal concentration, biomass dosage (L/S ratio) and temperature.
Materials and methods
Chromium of analytical standard was purchased from Merck India. Ultrapure water obtained using a Milli-Q UF-Plus apparatus (Millipore, MA, USA) was used to prepare all solutions for the method. All other chemicals used were of analytical grade unless otherwise stated. The microalgae, Isochrysis galbana, were collected from the Central Marine Fisheries Research Institute (CMFRI), Visakhapatnam. The chemicals used were of AR/LR grade supplied by different standard manufacturing industries or as indicated.
Preparation of stock solution
Chromium chloride solution was prepared by dissolving about 5.28 g of chromium chloride salt in a 1,000-mL standard volumetric flask with demineralized water. From the stock solution, experimental test solutions were prepared by diluting the primary stock solution with demineralized water. The pH of the test solution was altered by adding the appropriate amount of mineral acids.
Preparation of standard solutions for analysis
For atomic absorption spectrophotometer analysis, 1,000 ppm Merck standard chromium solution was used to prepare a standard solution. The primary standard solution was prepared by appropriate dilution of 1,000 ppm standard solution in accordance with the selected wavelength and sensitivity check. The concentrations of un-adsorbed chromium ions in the sample supernatant liquid were determined using an atomic absorption spectrophotometer (Perkin Elmer model AA 200) with an air–acetylene flame. CA and CS were then calculated and tabulated for subsequent analysis of the data. The metal uptake (CS) was calculated using the general definition:
where CS is the metal uptake of Cr(III) g−1 biomass, V is the volume of metal-containing solution in contact with the biosorbent in mL, CT and CA are the initial and equilibrium (residual) concentration of metal in the solution mg L−1, respectively, and M is the amount of added biosorbent on wet basis in L.
Metal percentage of removal by I. galbana was determined by Equation 2 as follows:
where R is the percentage of Cr(III) adsorbed by biomass, CT is the initial concentration of metal ions in mg L−1, and CA is the concentration of metal ions at time ‘t’ in mg L−1.
Preparation of algal beads
The microalga Isochrysis galbana was collected from the Central Marine Fisheries Research Institute (CMFRI), Visakhapatnam. I. galbana was (4 million/mL) rinsed twice with distilled water, filtered by vacuum filtration and resuspended in distilled water. Sodium alginate (5 %) solution was prepared with hot distilled water; at room temperature, equal quantities of algal solution and alginate solution were mixed thoroughly with a magnetic stirrer. The uniform mixture of alga and sodium alginate solution (2.5 %) was pumped through the peristaltic pump into the 0.5 M CaCl2 2H2O solution. The beads (2 million/mL) were stored at 4 °C overnight for curing with 0.25 M CaCl2 2H2O solution and washed twice with distilled water to avoid excess CaCl2 2H2O. These beads were used for equilibrium studies. Each mL of beads contained 0.0025 g of dry Isochrysis galbana biomass and this value was used for further calculation.
pH adjustment of aqueous metal solutions
The adsorption of metals decreases at low pH values because of competition for binding sites between cations and protons, while at pH > 7, hydroxo species of the metals can be formed and do not bind to the adsorption sites on the surface of the adsorbent. So, pH was adjusted to the range of 3–6 by adding the 0.1 N HCl and 0.1 N NaOH for acidic and basic pH, respectively. For chromium equilibrium studies with biomass, the pH was maintained in the range of 3–6 only, due to at the near to neutral pH and at basic pH the metal ions are precipitated.
Equilibrium studies
Each 100 mL of known concentration of chromium chloride solution was pipetted into a 250 mL cleaned stoppered conical flask and 10, 25 and 50 mL (L/S ratio 10, 4 and 2, respectively) volume of live I. galbana (beads) were added to the test solution separately. The volume of immobilized biomass was taken by replacement method at known wet weight of biomass. Equilibrium studies were carried out at 10, 30 and 50 °C temperature. The conical flasks were kept in the orbital shaker, which was set at 180 rpm and shaken for the required amount of time to attain equilibrium. Sample solutions were collected periodically and analyzed in the AAS for the metal content in the test solution after adsorption; the procedure was continued till the equilibrium conditions were obtained. Time required to attain equilibrium was thus established.
FTIR studies
The powdered biomass before and after adsorption was air dried and the moisture was removed completely at 60 °C in a humidity control oven. The powder was analyzed by Fourier transform infrared spectrophotometer (FTIR) by potassium bromide (KBr) pellet method in the wave number range of 400.00–4,000.00 cm−1 (Perkin-Elmer No. 72425).
Results and discussion
Effect of time
The time required to reach equilibrium was (Fig. 1) found to be 4½ h. 75 % of the Cr(III) was adsorbed in 2 h and almost 90 % in 4½ h. Further experiments were conducted for all other parameter estimations that were done at 4½ h. The rapid initial sorption was likely due to extra-cellular binding and the slower sorption resulted from intracellular binding (María and dos María 2010). This may be due to the embedded of algal biomass in the alginate beads; the diffusion barrier between the biomass and the outer diffusion layer of the bead takes a much longer time to reach the biomass surface. The direct naked biomass surface is easily available to complex with Cr(III) ions and takes less equilibrium time (Akhtar et al. 2008).
Effect of pH
The percent adsorption increased with increase in pH; a near pH 4.5 of the (Figs. 2, 5) solution was found to favor adsorption very strongly. At lower pH, the percentage adsorption was very low, maybe due to preferential adsorption of hydrogen ions. At low pH values (pH < 2.0), protons occupy most of the biosorption sites on the algal surface and less Cr(III) ions could be sorbed because of electric repulsion with the protons on the biomass. The pH was increased from 2 to 4.5, biomass surfaces were more negatively charged and the sorption of the metal ions with positive charge Cr(III) process was reached maximum at 4.5. Similar results were reported for Cr(III) on the other biomass (Akhtar et al. 2008).
Effect of L/S ratio
The effect of liquid solid ratio (L/S ratio) on percent adsorption for chromium (Fig. 3) using immobilized microalgae, I. galbana, as biosorbent material at fixed total metal concentration, pH 4.5 and 30 °C was investigated. It can also be observed that the increase in L/S ratio slightly decreases the percent adsorption, though the equilibrium concentration of metal uptake increases because more amount of algal beads are available in the given solution for adsorption as L/S ratio increases (Akhtar et al. 2008).
Effect of temperature
The temperature of the adsorption medium could be important for energy-dependent mechanisms in metal adsorption by algal cells. The effect of temperature on percent adsorption of chromium using immobilized I. galbana was observed (Fig. 4). A temperature of 50 °C was found to favor biosorption. The increase in adsorption with increasing temperature indicated the endothermic nature of the adsorption process. A similar endothermic nature of the adsorption process has been reported for other adsorbent systems (Aksu et al. 2002; Prakasham et al. 1999). The increase in sorption with temperature may be attributed to either increase in the number of active surface sites available for sorption on the adsorbent or to the decrease in the boundary layer thickness surrounding the sorbent, so that the mass transfer resistance of adsorbate in the boundary layer decreased (Meena et al. 2005; Gupta and Rastogi 2009). For an increase in temperature from 30 to 50 °C, the adsorption capacity of algal biomass for Cr(III) showed an increase from 95 to 98 % (Fig. 4), which is not a considerable extent of percent removal of Cr(III). Further, to increase the process temperature, more energy is required and it is expensive. Hence, a temperature of 30 °C is preferable for the biosorption process for Cr(III) on immobilized I. galbana biomass.
Effect of initial metal concentration
The amount of adsorbent significantly influenced the Cr(III) adsorption, i.e., the biosorption of metal ions decreased with increasing biomass dosage at pH 5 and was high at a dose of 10 mL I. galbana biomass beads (Fig. 5). This trend can be explained to be a consequence of partial aggregation of biomass at higher biomass beads, resulting in the decrease in effective surface area for biosorption (Karthikeyan et al. 2007; Gupta and Rastogi 2009). Percent removal of Cr(III) decreases with the increase in initial Cr(III) ion concentration. At lower concentration, all the Cr(III) in the solution would react with the binding sites and thus facilitate almost complete adsorption. At higher concentration, more Cr(III) ions are left un-adsorbed in the solution due to the saturation of the binding sites. This indicates that the energetically less favorable sites become involved with increasing Cr(III) ion concentration in aqueous solutions. A similar trend was observed in Cr(VI) removal on biomasses, i.e., rice straw, rice bran, rice husk, hyacinth roots, neem leaves and coconut shells (Biswajit and Sudip 2011).
Isotherms
Freundlich isotherm
An adsorption isotherm was proposed by Boedecker (Dabrowski 2001), which was later modified by Freundlich (Freundlich 1926). The Freundlich adsorption equation can be written as:
Taking logarithm on both sides,
where ‘qe’ is equilibrium adsorption capacity (mg g−1), ‘Ce’ is the equilibrium concentration of the adsorbate in solution, and ‘Kf’ and nf are constants related to the adsorption process such as adsorption capacity and intensity, respectively.
This empirical model has shown best fit for non-ideal sorption on heterogeneous surfaces as well as multilayer sorption. The Freundlich isotherm is also more widely used, but provides no information on the monolayer adsorption capacity, in contrast to the Langmuir model. Freundlich isotherm has been derived by assuming an exponentially decaying sorption site energy distribution.
The plot in Fig. 6 gives the isotherm drawn for biosorption of Cr(III) on to microalgae I. galbana. The coefficient of determination for this case is 0.976 and the values of nf and Kf (Table 1) are found to be 0.484 and 301.9 at 30 °C. This is also suggestive that the metal ion under study could well be separated from its aqueous solution with high adsorption capacity. The values of high correlation coefficients indicated that the Cr(III) sorption data were very well represented by the Freundlich model.
Langmuir isotherm
The equation proposed by Langmuir is universally applicable to chemisorption with some limitations involving physical adsorption. This equation is applicable to the physical or chemical adsorption on solid surface with one type of adsorption active center. As long as the limitations are clearly recognized, the Langmuir equation can be used for describing equilibrium conditions for adsorption behavior in different adsorbate–adsorbent systems for varied conditions within any given system. The linear form of the Langmuir equation is given by:
where qm is the maximum amount of the metal ion per unit weight of adsorbent that forms a complete monolayer on the surface bound at high Ce(mg g−1), and b is a constant which accounts for the affinity of the binding sites (L mg−1). qm represents the limiting adsorption capacity when the surface is fully covered with metal ions and helps in the evaluation of adsorption performance, particularly in cases where the sorbent did not reach its full saturation during contact. It is the most widely used simple two-parameter equation (Langmuir 1918), based on the assumption that adsorption cannot proceed as a monolayer coverage. There are a fixed number of adsorption sites at equilibrium. The isotherm showed good fit to the experimental data with good correlation coefficient (0.976). The qmax value of 392.65 mg g−1 of algal beads was nearer to the experimental value, i.e., 335.27 mg g−1. Nonlinear regression analysis was carried out in sigma plot 8.0 to determine the b value and shown in Table 1.
FTIR spectroscopic analysis
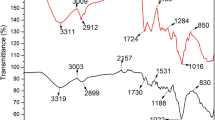
The FTIR spectroscopic analysis of the biomass, microalga I. galbana before adsorption of chromium metal ions (Fig. 7) showed several intense characteristic bands, which can be attributed to functional groups present in the protein and polysaccharides such as carboxylic, CH2, NH and –OH groups. The broad adsorption bands at 3,407.52–3 and 176.47 cm−1 represent –OH and –NH, respectively. The absorption band at 1,631.16 cm−1 can be attributed to the C=O group of carboxylic acid or amide bonds.
FTIR spectroscopic analysis of the biomass of microalga Isochrysis galbana after adsorption of heavy metal ion chromium showed that the –NH stretching was missing and a new peak at 3,132.42 cm−1 appeared (Fig. 8). It may be due to the interaction of –OH and –NH bonds. Some bands (472.84 cm−1) observed in the “fingerprint” region could be attributed to the phosphate group. Andrade et al. (2005) revealed that some groups present in lower levels in the algal cells were more difficult to detect with FTIR spectroscopic analysis and the same results were observed on freshwater alga Chaetophora elegans.
The significant changes in the wave numbers of the specific peaks suggested that hydroxyl, amide, bounded –OH, bounded –NH, C=H stretching vibrations, amide N–H bending vibrations and C=O of carboxylic acid groups could be predominantly involved in the biosorption of Cr(III) onto I. galabana. Similar results were reported for the biosorption of different heavy metals on various algal species (Sibel et al. 2009; Leila and Lassaad 2009; Suleman et al. 2009; Vítor et al. 2009; Rajesh et al. 2010; Munagapati et al. 2010).
Conclusions
-
1.
The time required to reach equilibrium was found to be 4½ h.
-
2.
Increase in aqueous metal concentration increased metal adsorption for all conditions of the study.
-
3.
Decreases in pH of aqueous phase decreased metal adsorption due to the preferential adsorption of H+ ion compared to metal ions, and pH 4.5 was found to favor metal adsorption.
-
4.
Increase in L/S ratio slightly decreases percent biosorption, though the equilibrium metal uptake increases.
-
5.
Increasing temperature increases metal adsorption in all cases of the study and in this process 30 °C is preferable.
-
6.
The maximum experimental biosorption capacity for entrapped live algal biomass was found to be 335.27 mg Cr(III) g−1 of dry algal biomass.
-
7.
Microalga Isochrysis galbana after adsorption of heavy metal ion chromium complexation occurred due to the interaction of –OH, –NH and C=O.
-
8.
Microalga Isochrysis galbana is a potential biosorbent for removal of chromium.
References
Akhtar N, Iqbal M, Zafar SI, Iqbal J (2008) Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). J Environ Sci 20:231–239
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium (II) biosorption by C. vulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294
Aksu Z, Acikel U, Kabasakal E, Tezer S (2002) Equilibrium modelling of individual and simultaneous biosorption of chromium(VI) and nickel(II) onto dried activated sludge. Water Res 36:3063–3073
Andrade AD, Rollemberg MCE, Nóbrega JA (2005) Proton and metal binding capacity of the green fresh water alga Chaetophora elegans. Process Biochem 40:1931–1936
Araújo AA, Andrade Santana MH (1996) Aerobic immobilized cells in alginate gel particles of variable density. Appl Biochem Biotechnol 57–58:43–55
Biswajit S, Sudip KD (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B Biointerf 84:221–232
Dabrowski A (2001) Adsorption—from theory to practice. Advan Colloids Interf Sci 93:135–224
Freundlich H (1926) Colloid and capillary chemistry. Methuen, London
Gülay B, Ilhami T, Gokce C, Meltem Y, Yakup AM (2006) Biosorption of mercury(II), cadmium(II) and lead(II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Inter J Mine Process 81:35–43
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interf Sci 271:321–328
Gupta VK, Rastogi A (2008) Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta K, Shrivastava AK, Neeraj J (2001a) Solutions by green algae Spirogyra species. Water Res 35(17):4079–4085
Gupta VK, Gupta M, Sharma S (2001b) Process development for the removal of lead and chromium from aqueous solutions using red mud an aluminium industry waste. Water Res 35(5):1125–1134
Gürü Metin, Venedik Duygu, Murathan Ayşe (2008) Removal of trivalent chromium from water using low-cost natural diatomite. J Hazard Mater 160:318–323
Hideshi S, Akira S, Yasuyuki I (2000) Biosorption of Heavy Metal Ions to A Marine Microalga, Heterosigma akashiwo (Hada) Hada. J Colloid Interf Sci 229:196–198
Karthikeyan S, Balasubramanian R, Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455
Kumar KK, Prasad MK, Sarma GVS, Murthy CVR (2009) Removal of Cd(II) from Aqueous Solution Using Immobilized Rhizomucor Tauricus. J Microb Biochem Technol 1:015–021
Kuyucak N, Volesky B (1998) Biosorbents for recovery of metals from industrial solutions. Biotechnol Lett 10:137–142
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lazaridis NK, Asouhidou DD (2003) Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg–Al–CO3 hydrotalcite. Water Res 37:2875–2882
Leila CA, Lassaad C (2009) Biosorption of Cu+2 and Zn+2 from aqueous solutions by dried marine green macroalga Chaetomorpha linum. J Environ Manage 90:3485–3489
Lloyd JR, Harding CL, Macaskie LE (1997) Tc (VII) reduction and accumulation by immobilized cells of Escherichia coli. Biotechnol Bioeng 55:505–510
María MA, dos María SA (2010) Copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus. thermodynamics and kinetics studies. Colloids Surf B Biointerf 81:620–628
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122:161–170
Mohan D, Singh KP, Singh VK (2006) Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater B 135:280–295
Munagapati VS, Yarramuthi V, Nadavala SK, Alla SR, Abburi K (2010) Biosorption of Cu(II), Cd(II) and Pb(II) by Acacia leucocephala bark powder: kinetics, equilibrium and thermodynamics. Chem Eng J 157:357–365
Patterson JB (1977) Waste water treatment. Science Publishers, New York, USA
Prakasham RS, Merrie JS, Sheela R, Saswathi N, Ramakrishna SV (1999) Biosorption of chromium(VI) by free and immobilized Rhizopus arrhizus. Environ Pollut 104:421–427
Rajesh S, Rout C, Rajender K, Kiran B, Divya B, Anil K, Narsi RB, Namita S (2010) Biosorption optimization of lead(II), cadmium(II) and copper(II) using response surface methodology and applicability in isotherms and thermodynamics modeling. J Hazard Mater 174:623–634
Sibel TA, Asli G, Burcu A, Zerrin K, Tamer A (2009) Investigation of the biosorption characteristics of lead (II) ions onto Symphoricarpus albus: batch and dynamic flow studies. J Hazard Mater 165:126–133
Smidsrød O, Skjåk -Braek G (1990) Alginate as immobilization matrix for cells. Tibtech 8:71–78
Suleman Q, Anwar RS, Muhammad U (2009) Biosorption of lead from aqueous solution by Ficus religiosa leaves: batch and column study. J Hazard Mater 166:998–1005
Tsezos M, Mccready RGL, Bell JP (1989) The continuous recovery of uranium from biologically leached solutions using immobilized biomass. Biotechnol Bioeng 34:10–17
Vítor JPV, Cidália MSB, José PSP, Rute FD, Rui ARB (2009) Copper removal by algal biomass: biosorbents characterization and equilibrium modeling. J Hazard Mater 163:1113–1122
Wu Y, Zhang S, Guo X, Huang H (2008) Adsorption of chromium (III) on lignin. Bioresour Technol 99:7709–7715
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas TL (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Acknowledgments
One of the authors, Mr. K. Kishore Kumar (SRF) is thankful to University Grants Commission, Delhi, Government of India (letter no. SA-II 10-47) for providing financial support and to UGC SAP/DSA Phase-III for permission to use the AAS. Further, our thanks are due to the Scientist In-Charge, Central Marine Fisheries Research Institute, Visakhapatnam for providing microalga Isochrysis galbana.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kadimpati, K.K., Mondithoka, K.P., Bheemaraju, S. et al. Entrapment of marine microalga, Isochrysis galbana, for biosorption of Cr(III) from aqueous solution: isotherms and spectroscopic characterization. Appl Water Sci 3, 85–92 (2013). https://doi.org/10.1007/s13201-012-0062-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0062-1