Abstract
Treatment of textile dye wastewater was carried using Pleurotus floridanus in a batch reactor. Response surface methodology (RSM) was used to optimize the process parameters like pH, temperature, agitation speed and dye wastewater concentration for the decolorization of textile dye wastewater. The optimum conditions for the maximum decolorization was: pH 6.6, temperature 28.8 °C, agitation speed 183 rpm and dye wastewater concentration 1:2. From the results it was found that, the linear effect of agitation speed and initial textile dye wastewater concentration were more significant than other factors for the textile dye wastewater treatment. At these optimized conditions, the maximum decolorization and COD reduction was found to be 71.2 and 80.5 %, respectively. Kinetics of textile dye degradation process was studied by various models like first order, diffusional and Singh model. From the results it was found that the degradation follows first order model with R2 value of 0.9550.
Similar content being viewed by others
Introduction
Dyes are synthetic, complex, ionizing, aromatic structures that are responsible for their stability against light, sweat, heat, chemical oxidant, etc. (Aksu and Tezer 2005). Different classes of synthetic dyes are widely used as coloring agent in textile, paint, ink, plastic industries (Zollinger 1991). It was estimated that, around 10–15 % of dyes are released out during dyeing process and mixed with water bodies and produce serious impact on environment. The colored textile wastewater is considered to be highly toxic with presence of organic contaminants and chemicals. They create odor, bad taste, foaming etc. and are toxic to aquatic life, reduce photosynthesis, carcinogenic, mutagenic (Malik and Taneja 1994) and some of them make allergy, dermatitis and skin irritation to human (Wesenberg et al. 2003).
Many physiochemical processes have been developed for the removal of color and pollutants present in the textile dye wastewater. Adsorption, chemical oxidation and reduction, chemical precipitation, flocculation, photolysis etc. are some of the methods commonly used for the decolorization of dye wastewater (Ansari and Thakur 2006; Zhang et al. 2002; Rajeshkannan et al. 2010, 2011). These methods are mostly ineffective, expensive and produce side reactions, high sludge and by products formation and not suited to degrade all dyes, etc. (Krull et al. 1998; Verma and Madamwar 2003). Hence the researchers focused on biological treatments as the best alternative due to their low operational cost (Arutchelvan et al. 2003; Jadhav and Govindwar 2006). Many microorganisms have been reported for their ability to decolorize the dyes include bacteria, fungi, actinomycetes etc. (Chang et al. 2001; Khehra et al. 2005). Among these microorganisms, white rot fungi are the most intensive studied dye decolorizing microbes and it produce large quantity of extra cellular enzymes that helps to remove dyes from industrial effluent and also they have ability to resist unfavorable environmental conditions (Bumpus 1998; Pointing 2001).
In this study, a white rot fungal strain, Pleurotus floridanus was used to treat the textile dye industry wastewater. The effect of process variables on textile dye industry wastewater degradation was studied and optimized using response surface methodology (RSM).
Materials and methods
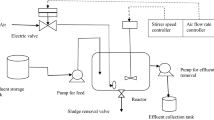
The textile dye wastewater was collected from a private small scale industry located at Erode, Tamil Nadu, India. The waste water was analyzed for various parameters and it was given in Table 1. The wastewater was stored at 4 ± 1 °C in air tight plastic containers. Pleurotus floridanus (MTCC-6315) is a stock of the microbial type culture collection centre (MTCC), Chandigarh, India. It was well preserved in the laboratory. The strain was maintained on solid medium at 4 °C. The medium composition and conditions were: Malt extract 20 g/L; Agar 20 g/L; pH 6.5; temperature 25 °C. The subculture was made at regular intervals of 30 days. The liquid culture used in the experiments was prepared from stock culture using sterilized malt extract medium. The pH of the medium was adjusted using 0.1 N HCl and 0.1 N NaOH in 250 ml Erlenmeyer flasks and allowing it to grow for 10 days at 25 °C to become active mycelia growth.
Response surface methodology (RSM)
In this work, Box–Behnken design was used to study the effects of the variables towards their responses and for optimization studies. This method is suitable for fitting a quadratic surface and it helps to optimize the effective parameters with a minimum number of experiments, as well as to analyze the interaction between the parameters. A regression design is normally employed to model a response as a mathematical function of a few continuous factors and good model parameter estimates are desired.
The coded values of the process parameters are determined by the following equation
where xi coded value of the ith variable, Xi uncoded value of the ith test variable and X0 uncoded value of the ith test variable at center point.
The regression analysis was performed to estimate the response function as a second order polynomial
Where Y is the predicted response, β i , β j , β ij are coefficients estimated from regression. They represent the linear, quadratic and cross products of x1, x2, x3 on response.
The regression and graphical analysis with statistical significance were carried out using Design-Expert software (version 7.1.5, Stat-Ease, Inc., Minneapolis, USA). In order to visualize the relationship between the experimental variables and responses, the response surface and contour plots were generated from the models. The adequacy of the models was further justified through analysis of variance (ANOVA).
Experimental procedure
The range and levels of process parameters, pH, temperature, agitation speed and dye wastewater concentration were given in Table 2. According to Box–Behnken design (Table 3), experiments were performed in a 500 cc Erlenmeyer flask. pH was adjusted (5, 6, 7) using 0.1 N HCl and 0.1 N NaOH. Decolorization was monitored in an UV spectrophotometer (model: BL-200, ELICO, India) by changing the absorbance of sample. Samples were withdrawn and centrifuged at 10,000 rpm for 10 min to remove fungal mycelia. The pellet was discarded after centrifugation and clear solution was analyzed in the UV spectrophotometer at 395 nm. COD of the samples was analyzed using the procedure given in APHA (1998).
Results and discussion
The effect of process parameters, on the decolorization and COD reduction of textile dye wastewater were studied. The second order polynomial coefficients for each term of the equation (Eqs. 3, 4) were determined using the Design-Expert 7.1.5. The experimental and predicted values of percentage decolorization and degradation were given in Table 3.
where A, B, C and D were the coded values of the process variables, pH, temperature (°C), agitation speed (rpm) and wastewater concentration, respectively.
The results were analyzed by using analysis of variance (ANOVA) and were given in Table 4. The ANOVA of the quadratic regression model indicates the model is significant. In this work, the model F value 19.79 and 34.04 for decolorization and COD reduction implies that the models were significant. The smaller the magnitude of the P, more significant is the corresponding coefficient. P value less than 0.05 indicate the model terms are significant. From the P values it was found that, the variables, C, D, A2, C2, D2 were significant model terms for decolorization and C, D, AD, BC A2, D2 were significant model terms for COD reduction. From the ANOVA table it was found that the linear effect of dye wastewater concentration is more significant for textile dye wastewater treatment followed by agitation speed.
The predicted R2 of 0.8354 (decolorization), 0.8634 (COD reduction) was in reasonable agreement with the adjusted R2 of 0.9428 (decolorization) and 0.9495 (COD reduction). The fit of the model is also expressed by the coefficient of regression R2, which is found to be 0.9714 for decolorization and 0.9747 for COD reduction, indicating that more than 97 % of the variability in the response could be explained by the model. This implies that the prediction of experimental data is quite satisfactory. The magnitude of coefficient factors in Table 4 gives the positive contribution of pH, agitation speed and wastewater concentration and negative contribution of temperature on dye wastewater decolorization and COD reduction.
To investigate the interactive effect of two factors on the decolorization and degradation of textile dye wastewater, response surface methodology was used and 3D plots were drawn. Response surface plots as a function of two factors at a time, maintaining all other factors at fixed levels are more helpful in understanding both the main and the interactive effects of two factors. The response surface curves for the decolorization and degradation of textile dye wastewater were shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12. The nature of the response surface curves shows the interaction between the variables. The elliptical shape of the curve indicates good interaction between the two variables and circular shape indicates no interaction between the variables. From the figures it is observed that the elliptical nature of the contour in graphs depicts the mutual interactions of all the variables. There is a relative significant interaction between every two variables, and there is a maximum predicted yield as indicated by the surface confined in the smallest ellipse in the contour diagrams.
Figure 1 shows the interactive effect of pH and temperature on textile dye decolorization. pH is one of the important factor in the treatment of textile dye wastewater by microorganism. From the figure, it was inferred that increase in pH (up to 6.6) increases the dye decolorization efficiency. After that the decolorization efficiency decreases. Similar trend was observed in Figs. 2 and 3. The pH has a major effect on the efficiency of dye decolorization, and the optimal pH for color removal is often between 6.0 and 7 for most of the dyes.
From Fig. 1, it is also observed that the decolorization increases with temperature up to 28.8 °C and thereafter decreases. Decolorizing activity was significantly suppressed at higher temperatures. This may be due to the loss of cell viability or deactivation of the enzymes responsible for decolorization. From Fig. 2, it was observed that, the percentage of decolorization increases with increase in agitation speed up to 183 rpm. After that point, the dye removal efficiency decreases. This was also observed in Figs. 4 and 6. The decrease in dye concentration increases the decolorization. This is clearly depicted in Figs. 3, 5 and 6. From the figures it is inferred that the percentage removal of dyes increases with decrease in dye concentration. This is because at higher concentrations the chemicals and other pollutants present in the dye wastewater inhibit the growth of microorganism. Similar trends were observed for the effect of process variables on COD reduction of textile dye wastewater and they were depicted in Figs. 7, 8, 9, 10, 11, 12.
The second order polynomial equation obtained from RSM was used to find the optimum conditions. Equation was solved in MATLAB 7.0. The optimum condition for the maximum decolorization was found to be: pH 6.6, temperature 28.8 °C, agitation speed 183 rpm and dye wastewater concentration 1:2. The optimal conditions predicted using RSM has been validated using experiments. At the optimized condition, the maximum color removal and COD reduction were found to be 71.2 and 80.5 %, respectively.
At the optimum condition, decolorization of textile wastewater was studied by analyzing the supernatants at different time intervals, in a UV spectrophotometer in the range of 300 to 800 nm. The results obtained were shown in Fig. 13. A peak is observed at λmax 395 nm in the UV–Vis spectra. The peak decreases as the day progresses, which showed the decolorization of textile dye using Pleurotus floridanus at the end of 5th day of operation.
Kinetics
In this study, first order model, diffusional model and Singh model were tried to fit the experimental data obtained from the batch degradation of textile dye wastewater using Pleurotus floridanus.
First order model
The first order model is
on integration between known limits and rearranging, the above model becomes
where Cso initial substrate concentration (mg COD/L), Cs substrate concentration (mg COD/L), t degradation time (h), k1 first order rate constant (h−1).
Diffusional model
The diffusional model is given by
when integrated between the known limits, the above equation becomes
Where k2 = rate constant for diffusional model.
Singh model
The Singh model is given by
integrating the above equation between the proper limits, it becomes
where k3 rate constant for Singh model.
The data obtained from the batch study were fitted to first order model, diffusional model and Singh model and it was shown in Figs. 14, 15 and 16, respectively. The rate constants, k1, k2 and k3 were calculated from the slope of the straight line by the least square (LSQ) fit in the figures. The detailed results including the determination coefficient (R2) were presented in Table 5. The high R2 value for the first order indicates the fitness of the model for the degradation of textile dye wastewater. The negative value of the rate constant for diffusional model and low R2 value for Singh models shows the inability of these models in describing this process.
Conclusions
In this work, a white rot fungus, Pleurotus floridanus was utilized to treat the textile dye wastewater. The process parameters pH, temperature, agitation speed and dye wastewater concentration were optimized using RSM. At the optimized condition, a maximum of 71.2 % color removal and 80.5 % COD reduction occurs. The UV spectrum confirms the decolorization of textile dye wastewater by Pleurotus floridanus. Kinetics of the textile dye wastewater degradation process was studied by various models. From the results it was found that the degradation follows first order. From the results it can be concluded that Pleurotus floridanus can be effectively utilized for the treatment of textile dye wastewater.
References
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40:1347–1361
Ansari AA, Thakur BD (2006) Biochemical reactor for treatment of concentrated textile effluent. Colourage 2:27–31
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association (APHA), Washington
Arutchelvan V, Albino DJ, Muralikaishnan V, Nagarajan S (2003) Decolourization of textile mill effluent by Sporotrichum pulverulentum. Indian J Environ Ecoplaning 7:59–62
Bumpus JA (1998) In: Bioremediation: principles and practice. Sikdar SK, Irvine RL, Vol 2, Technomic Publishing Company, Inc., Lancaster, pp 71
Chang JS, Chou C, Chen SY (2001) Decolorization of azo dyes with immobilized Pseudomonas luteola. Process Biochem 36:757–763
Jadhav JP, Govindwar SP (2006) Biotransformation of malachite green by Saccharomyces cerevisiae. Yeast 23:315–323
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimmi SS (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigments 67:55–61
Krull R, Hemmi M, Otto P, Hempel DC (1998) Combined biological and chemical treatment of highly concentrated residual dye house liquors. Water Sci Technol 38:339–346
Malik A, Taneja U (1994) Utilizing fly ash for color removal of dye effluents. Am Dyestuff Rep 83:20–27
Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 57:20–33
Rajeshkannan R, Rajasimman M, Rajamohan N (2010) Optimization, equilibrium and kinetic studies on removal of acid blue 9 using brown marine algae Turbinaria conoids. Biodegradation 21:713–727
Rajeshkannan R, Rajasimman M, Rajamohan N (2011) Sorption of acid blue 9 using Hydrilla verticillata biomass—optimization, equilibrium, and kinetics studies. Bioremediation 15:57–67
Verma P, Madamwar D (2003) Decolorization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J Microbiol Biotechnol 19:615–618
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Zhang F, Yediler A, Liang X, Kettrup A (2002) Ozonation of the purified hydrolyzed azo dye reactive red 120 (CI). J Environ Sci Health A Tox Hazard Subst Environ Eng 37:707–780
Zollinger H (1991) Colour chemistry: synthesis properties and application of organic dyes and pigments. VCH Publishers, New York
Acknowledgments
The authors wish to express their gratitude for the support extended by the authorities of Annamalai University, Annamalai Nagar, India in carrying out the research work in Environmental Engineering Laboratory, Department of Chemical Engineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sathian, S., Radha, G., Shanmugapriya, V. et al. Optimization and kinetic studies on treatment of textile dye wastewater using Pleurotus floridanus. Appl Water Sci 3, 41–48 (2013). https://doi.org/10.1007/s13201-012-0055-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0055-0