Abstract
The study comprises the use of cyanobacterial mat (collected from tannery effluent site) to remove hexavalent chromium. This mat was consortium of cyanobacteria/blue-green algae such as Chlorella sp., Phormidium sp. and Oscillatoria sp. The adsorption experiments were carried out in batches using chromium concentrations 2–10, 15–30 and 300 ppm at pH 5.5–6.2. The adsorption started within 15 min; however, 96 % reduction in metal concentration was observed within 210 min. The adsorption phenomenon was confirmed by Fourier transform–infrared spectroscopy and energy dispersive X-ray analysis. This biosorption fitted Freundlich adsorption isotherm very well. It was observed that the best adsorption was at 4 ppm, and at 25 ppm in the chosen concentration ranges. Scanning electron micrograph showed the physiology of mat, indicating sites for metal uptake. The main focus was collection of the cyanobacterial mat from local environments and its chromium removal potential at pH 5.5–6.2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discharge of chromium (VI) into aquatic ecosystems has become a matter of concern in all the tannery areas over the last few decades. This pollutant is introduced into the aquatic systems significantly from the chrome tanning effluents of leather processing units. In this process about 60–70 % of chromium reacts with the hides and about 30−40 % of chromium remains in the solid and liquid wastes (especially as spent tanning solutions). Chromium (VI) has been designated as the priority pollutant by United States Environment Protection Act (Srinath et al. 2002; Laxman and More 2002).
Conventionally, chromium (VI) containing industrial effluent is treated by physico-chemical methods such as reduction, precipitation, ion exchange, reverse osmosis and electrodialysis. However, it has been observed that these processes are costly and unreliable (Camargo et al. 2005).
Presently, biotechnological methods are becoming attractive alternatives to remove toxicants from effluents. The ability of some microorganisms especially cyanobacteria to tolerate and interact with metal/chromium ions makes them attractive substrate for environmental biotechnology (Cervantes et al. 2001). Cyanobacteria-based bioremediation technologies have received recent attention as strategies to clean up contaminated soil and water. The microbial mats containing several microbial species are complex systems, but require few external inputs to be exploited for bioremediation. Several workers have shown the use of different biomaterials such as non-living bacteria, microalgae, fungi, seaweed and even agricultural byproducts, which can retain relatively high quantities of metal ions by passive sorption and/or by complexation mechanism.
It has been shown that the short-term uptake/accumulation of chromium (VI) by interactions with cyanobacteria, Anabaena variabilis and Synechococcus PCC 6301, using their ability to sequester chromium (Garnham and Green 1995).
Aksu and Balibek (2007) showed the biosorption of chromium (VI) by dried Rhizopus arrhizus and its effect on addition of salt (NaCl). Arica et al. (2005) demonstrated biosorption of chromium (VI) from aqueous solutions using free and immobilized biomass prepared from Lentinus sajor-caju and studied the kinetic characterization. Thus, fungal species are also efficient in removing chromium metal. Biosorption of the chromium ion chromium (VI) onto the cell surface of non-pathogenic species of Trichoderma fungal species in aerobic condition at pH 5.5 gave 97.39 % reduction (Vankar and Srivastava 2008). Arica and Bayramoglu (2005) also showed the utilization of native, heat and acid-treated microalgae Chlamydomonas reinhardtii for biosorption of chromium (VI) ions. Deepa et al. (2006) have shown the sorption of chromium (VI) from dilute solutions and wastewater by live and pretreated biomass of Aspergillus flavus. Similarly, Zhou et al. (2007) have demonstrated the kinetic and equilibrium studies of chromium (VI) biosorption by dead Bacillus licheniformis biomass.
In the present study, the work has been carried out using a cyanobacterial mat (collected from tannery effluent disposal site) to remove chromium (VI) from tannery effluent at relatively higher pH, i.e., 5.5–6.2. The consortium of bacteria present was Chlorella sp., Phormidium sp. and Oscillatoria sp. (cyanobacteria/blue-green algae). The main focus was hexavalent chromium removal potential of cyanobacterial mat collected from the local environment at relatively higher pH 5.5–6.2.
This is the first report where chromium (VI) removal has been carried out at relatively higher pH so efficiently, although it is known that at low pH (0–2) the biosorption of chromium (VI) is more predominant than bioreduction of chromium (VI) to (III). On the other hand, at higher pH such as 3.0 and above, less of chromium (III) species were known to be produced due to the sharp decrease in both biosorption and bioreduction processes (Han et al. 2008). Some researchers have reported that the optimal pH for total chromium biosorption was around 2–3. The optimal pH for Sphagnum-moss peat, leaf, mould and coconut-husk fiber were reported to be 1.5, 2.0, 2.05 and 2.0, respectively while 2–2.5 was the optimal pH reported for Sargassum (Kratochvil et al. 1998; Cabatingan et al. 2001).
Seven exopolysaccharide-producing cyanobacteria were tested with regard to their capability to remove Cr(VI) from the wastewater of a plating industry. The cyanobacterium which showed, under lab conditions, the most promising features with regard to both Cr(VI) removal (about 12 mg of Cr(VI) removed per gram of dry biomass) and growth characteristics (highest growth rate and simplest culture medium) was Nostoc PCC7936 (Colica et al. 2010).
Very recently a study exploring the utilization of waste cyanobacterial biomass of Nostoc linckia from a lab-scale hydrogen fermentor for the biosorption of Cr(VI) from aqueous solution has been reported (Sharma et al. 2011). The biomass immobilized in alginate beads was used for removal of the metal in batch mode optimizing the process conditions adopting response surface methodology (RSM). Kinetic studies were done to get useful information on the rate of chromium adsorption onto the cyanobacterial biomass, which was found to follow pseudo second-order model.
Experimental
Cyanobacterial mat
The cyanobacterial mat was obtained from a disposal site near tannery sludge in Jajmau tannery area in Kanpur. The Tannery effluent was of strongly acidic nature (pH 3–4) with TDS ranging from 850 to 1,000 and salinity toward 600–700 PPT. The mat contained three species of bacteria, i.e., Chlorella sp. Phormidium sp. and Oscillatoria sp. as identified by Dr R.D. Tripathi, Taxonomer, National Botanical Research Institute, Lucknow.
Reagents
Metal stock solution (1,000 ppm) was prepared by potassium dichromate salt of Merck AR grade and deionized water (DI). It was then diluted to required concentration 2–10, 15–30 and 300 ppm by DI. The standard calibration was also carried out.
Biosorption methodology
In this study, batch biosorption experiments were carried out in triplicates for determining the biosorption capacity of cyanobacterial mat under aerobic conditions. Before the biosorption experiments, the cyanobacterial mat was desorbed for 30 min in 0.1 N HCl. An acclimatization period of 30 min was given for every batch study. Aqueous chromium (VI) solution with initial concentrations (2–10, 15–30 and 300 ppm) was prepared and 40 ml of each solution was taken and the pH was maintained between 5.5 and 6.2 to which the weighed amount (1.5 gm) of wet cyanobacterial mat was poured. The batch was then kept at room temperature, samples were withdrawn at different time intervals from it for colorimetric determination of chromium (VI) by Thermo Heλios α model spectrophotometer and percentage reduction in chromium (VI) concentration was calculated.
Treatment of cyanobacterial mat with EDTA
A new set of biosorption study was set up by pretreatment of cyanobacterial mat. The cyanobacterial mat was treated with N/56 EDTA for 15 min. Biosorption study was carried out by treated mat to compare the results.
Estimation of chromium VI by DPC method
Principle
Diphenyl carbazide reagent combines with hexavalent chromium to form a complex which gives a beautiful pink/magenta color in acidic medium as described in the “Method” (ISO Wq 1994).
Reagents
-
1.
Standard solution of potassium dichromate (2–10, 15–30 and 300 ppm).
-
2.
Diphenyl carbazide solution (0.1 % w/v) in acetone.
-
3.
3.1 N Hydrochloric acid solution.
Method
All the samples were filtered first through common filter paper then by Whatman No. 42 and finally by membrane filter (Millipore, 0.45 μm). The pH of the filtrate was maintained between 1 and 1.3, and to this solution 1 ml of DPC solution was added and the solution was shaken, readings were taken after 10 min on spectrophotometer at the requisite wavelength 540 nm.
Scanning electron microscopy and EDX
The control and adsorbed samples of cyanobacterial mat of size 1 × 1 mm were used for SEM and EDX. Scanning electron micrographs were taken on FEI Quanta 200 and EDX was recorded on Genesis Spectrum Ver. 3.6 (EDAX Inc.).
Adsorption isotherm
The sorption data have been subjected to different sorption isotherms, namely the Freundlich and Langmuir. The data fitted well to Freundlich isotherm well. The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution. The empirical model was shown to be consistent with exponential distribution of active centers characteristic of heterogeneous surfaces. The Freundlich adsorption isotherm was tested in the following linearized form:
by plotting log Qe versus log Ce. Ce is the equilibrium concentration of metal ions and Qe is metal ion adsorbed per unit mass of adsorbent (mg/g). Both Kf and n are constants, being indicative of the extent of adsorption and the degree of non-linearity between solution and concentration, respectively. The result is represented in Tables 2 and 3. From the slope and intercept of straight portion of the plot, the values of Freundlich parameters, i.e., 1/n and Kf are computed, the adsorption isotherm experimental data obtained was fitted to Freundlich isotherm. The magnitude of Kf and n shows easy separation of heavy metal ions from wastewater and high adsorption capacity. The value of n, which is related to the distribution of bonded ions on the sorbent surface, represents beneficial adsorption if it is between 1 and 10. The n value for the biosorbent used was found (Kadirvelu and Namasivayam 2000) to be >1, indicating that adsorption is favorable. Tables 2 and 3 give the isotherm parameters for Freundlich isotherms. From linear correlation coefficients of the adsorption isotherm, it can be said that sorption data is obeyed well, which is indicative that multilayer sorption takes place on the surface of cyanobacterial mat.
Results and discussion
In recent years, there has been an increasing research interest in microorganisms that are able to transform the highly toxic and water-soluble chromium (VI) to the less toxic and insoluble chromium (III) or remove chromium (VI) by adsorption. Considering the versatility of cyanobacteria and their ability to survive under diverse environmental condition, strains originating from different habitats must be studied with respect to their applicability (Hameed and Hasnain 2005). Many microorganisms are known to reduce chromium (VI) to chromium (III). Mats sequester or precipitate metals by surface adsorption or by conditioning the surrounding chemical environment, thus bio concentrating the metal in a small volume.
Cyanobacteria are the member of morphologically diverse group of prokaryotes. The oxygenic photosynthetic pathway adopted by such prokaryote is the presence of two photosystems (PSII and PSI), and the use of H2O as the photoreductant in photosynthesis (Tamagnini et al. 2002; Boone et al. 2001) is well known. Mats display wide variety of mechanisms for removal of metals and metalloids. These mechanisms take place at the cellular level of the constituent microorganism and at the community level of entire consortium.
The biosorption experiments were carried out in batches with standard chromium concentration (2–10, 15–30 and 300 ppm) and pH of 5.5–6.2. The percentage of reduction in the chromium (VI) concentration by cyanobacterial mat at different time periods are shown in Table 1. A totally new phenomenon was observed in the present case. The substantial biosorption that occurred at initial pH 5.5–6.2, could be attributed to the fact that the mat itself was releasing H+ during the process and thus the pH had been observed to have lowered with time duration.
In this study, the pathway adapted for biosorption by the cyanobacterial mat seems to be chromate reduction. The probable mechanism of reduction could be envisaged as following: (1) the participation of photosynthesis by the Chlorella taking place in the upper cyanobacteria stratum which provided a continuous supply of electron for the chromium (VI) to (III) reduction process, (2) the consortium was able to partially adsorb chromium as well as to partially reduce chromium (VI) to chromium (III), and (3) at very high chromium (VI) concentration (300 ppm) the mat showed very efficient removal. This indicated that chromium (VI) was first getting adsorbed and then the adsorbed metal species were getting reduced by the bioreductant released by the mat.
In most of the cases the mat showed 100 % chromium (VI) removal in overnight, except in the cases of 4 and 6 ppm, where it showed total chromium (VI) removal in 90 and 210 min, respectively. In other cases adsorption of this metal ion was above 90 %, i.e., 91.45, 96.85 and 95.16 % for 2, 8 and 10 ppm, respectively. The short-term uptake of chromate (biosorption) was concentration dependent and the data were found to conform to the Freundlich adsorption isotherm as shown in Tables 2, 3.
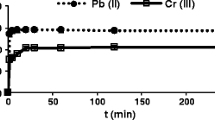
Another set of chromium biosorption with higher concentration of chromium, i.e., 15, 20, 25 and 30 ppm was also carried out as shown in (Fig. 1).
In this set an average removal of chromium (VI) has been observed except in the case of 15 ppm where the removal was 94.76 % in 210 min. However, after overnight the mat showed 100 % chromium (VI) removal in every batch. It was observed that best adsorption among the lower concentration range was at 6 ppm while for higher concentration good adsorption was observed at 25 ppm. This could be attributed to low acclimatization for higher concentration of chromium (VI) ion for the mat.
In another study, cyanobacterial mat was treated with EDTA to check the effect of treatment on metal removal by the mat. The mat was suspended in EDTA to increase cellular apertures so that cell surface could be increased. These experiments were carried out with 2 and 10 ppm standard dichromate solution. EDTA treatment did not show any positive effect on the removal/reduction of chromium (VI) as compared to control set. The results did not show the expected trend of higher reduction for the EDTA treated mat. The untreated mat seemed to be more efficient in metal removal.
This bacterial consortium was also tested against 300 ppm of chromium (VI) concentration and it showed efficient removal of chromium by 1.5 g of the mat. This indicates that surface area is not a limiting factor and both biosorption and bioreduction are occurring simultaneously.
Scanning electron micrograph, shown in Figs. 2 and 3, respectively, explains the morphology of mat before and after biosorption.
It was found to be a well-established mat containing Oscillotoria sp., Phormidium sp. and Chlorella sp. The entire network was a mesh containing Chlorella sp. on upper surface of the mat, while Oscillotoria sp. and Phormidium occupied the lower side. The cyanobacterial physiology did not get affected by the presence of high concentrations of chromium possibly due to the fact that the mat surface could be highly negatively charged due to the hydrolysis of the surface polysaccharides causing more number of hydroxyl ions to be present on the mat surface. The charges would be possibly distributed along the filamentous cyanobacteria which would have provided an enormous surface area for binding of positively charged chromium ions. Thus, it was the ability of the entire mat surface which would be eventually responsible for higher sequestering of chromium (VI) ion.
The actual mechanism of the initial chromium (VI) reduction by the mat is not known. Chromate reductase has been identified in other microbial groups which demonstrate both aerobic and anaerobic reduction of chromium (VI) as demonstrated by Tamagnini et al. (2002) and Castenzholz et al. (2001). However, the presence of chromate reductase has not been investigated in the cyanobacterial mat.
FT-IR spectra of the mat after chromium (VI) biosorption showed four apparent changes of the functional groups on the biomass. (1) The enhancement of the intensity at the region 3,200–3,500 cm−1 indicates an increase of the free hydroxyl group on the biomass (Fig. 4). This could be due to hydrolysis of some polysaccharides on the cell wall to shorter saccharides such as oligosaccharides, dioses and monoses under acidic condition as observed (Lin et al. 2005). (2) The weakening of the peak at 1,470–1,450 cm−1, which was typical of the complexation of the carboxylate functional group by coordination with chromium (III) metal formed from bioreduction of chromium (VI). (3) The third change was the shift of the peak at 1034–1028 cm−1, which could be due to the involvement of the C–O bond of polysaccharides in chromium (III) biosorption. (4) The last change was the presence of a new peak at around 940 cm−1 in the chromium (VI)-treated biomass, and it could be attributed to the presence of chromium (VI)–O bond as suggested (Holman et al. 1999).
During the metal remediation study it was also observed that the mat secreted purple colored dye identified as phycoerythrin by UV–Visible spectrum having a peak at 550 nm. It is known that Phormidium possess phycocyanin such as phycoerythrin. Phormidium sp. cells containing phycoerythrin has been reported to show a single absorption peak at 565 nm in the visible wavelength region (Haxo 1960).
The EDX data of mat and biosorbed mat shown in Figs. 5 and 6 respectively clearly showed the enhancement of chromium content in the biosorbed mat. The mat initially contained 29.68 % of chromium along with other metal ions as it was collected from effluent disposal site and the mat after biosorption contained 47.25 %. A net increase of 17.57 % in chromium content was observed.
Based on the results, it is reasonable to conclude that the mechanism involved in the removal of chromium (VI) by cyanobacteria mat was found to biosorption–bioreduction. The sequence of reaction that may be occurring is probably (1) the protons released by the mat (from the photosynthetic pathway of Chlorella) would be creating protonated sites on the mat for biosorption of chromium (VI), (2) bioreduction of chromium (VI) to chromium (III), the chromium (VI) adsorbed on the biomass surface was bioreduced to chromium (III) by the reductant on the biomass such as polysaccharides and the electrons generated during the photosynthesis carried out by Chlorella. Phormidium and the other microbes produce hydrogen sulfide that leads to anoxic conditions. It has been observed that elemental sulfur (showed positive test for sulfate ion) was deposited which seems to have come from anaerobic environments with abundant hydrogen sulfide produced by Phormidium and in the presence of light, Oscillatoria are known to undergo an oxygenic photosynthesis to excrete elemental sulfur rather than oxygen gas. Thus, the entire consortium was found to be participated in the biosorption process.
Conclusions
Although cyanobacterial mats occur in nature as stratified communities of cyanobacteria and some other bacteria, but they can be cultured on large scale and used for bioremediation. This study is an example of bioremediation where a cyanobacterial mat is used in form of biological treatment to clean up chromium (VI) contaminant in surface water at pH 5.5–6.2 for both low and high levels of contamination. Moreover, it offers permanent in situ remediation rather than simply moving the pollution to other site. One of the important facts about this study was, due to possible release of acid by the mat during the biosorption, the process could easily be carried out at higher initial pH 5.5–6.2. Thus, the cyanobacterial mat, which is genetically and ecologically modified species, seems to be potential savior for the chromium (VI) pollution caused by the tannery industry.
References
Aksu Z, Balibek E (2007) Chromium(VI) biosorption by dried Rhizopus arrhizus: Effect of salt (NaCl) concentration on equilibrium and kinetic parameters. J Hazard Mater 145(1–2):210–220. doi:10.1016/j.jhazmat.2006.11.011
Arica MY, Bayramoglu G (2005) Cr(VI) biosorption from aqueous solutions using free and immobilized biomass of Lentinus sajor-caju: preparation and kinetic characterization. Colloids Surf, A 253(1–3):203–211. doi:10.1016/j.colsurfa.2004.11.012
Arica MY, Tüzün I, Yalçin E, Ince Ö, Bayramoglu G (2005) Utilisation of native, heat and acid-treated microalgae Chlamydomonas reinhardtii preparations for biosorption of Cr(VI) ions. Process Biochem 40(7):2351–2358. doi:10.1016/j.procbio.2004.09.008
Boone DR, Castenholz RW, Garrity GM (eds) (2001) Bergey’s manual of systematic bacteriology, vol 1, 2nd edn. Springer, New York, p 173
Cabatingan LK, Agapay RC, Rakels JLL, Ottens M, van der Wielen LAM (2001) Potential of biosorption for the recovery of chromate in industrial wastewaters. Ind Eng Chem Res 40(10):2302–2309. doi:10.1021/ie0008575
Camargo FAO, Okeke BC, Bento FM, Frankenberger WT (2005) Diversity of chromium-resistant bacteria isolated from soils contaminated with dichromate. Appl Soil Ecol 29(2):193–202. doi:10.1016/j.apsoil.2004.10.006
Cervantes C, Campos-Garcia J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25(3):335–347. doi:10.1016/s0168-6445(01)00057-2
Colica G, Mecarozzi PC, Philippis RD (2010) Treatment of Cr(VI)-containing wastewaters with exopolysaccharide-producing cyanobacteria in pilot flow through and batch systems. Appl Microbiol Biotechnol 87:1953–1961
Deepa KK, Sathishkumar M, Binupriya AR, Murugesan GS, Swaminathan K, Yun SE (2006) Sorption of Cr(VI) from dilute solutions and wastewater by live and pretreated biomass of Aspergillus flavus. Chemosphere 62(5):833–840. doi:10.1016/j.chemosphere.2005.04.087
Garnham GW, Green M (1995) Chromate (VI) uptake by and interactions with cyanobacteria. J Ind Microbiol Biotechnol 14(3):247–251. doi:10.1007/bf01569935
Hameed A, Hasnain S (2005) Cultural characteristics of chromium resistant unicellular cyanobacteria isolated from local environment in Pakistan. Chin J Oceanol Limnol 23(4):433–441. doi:10.1007/bf02842688
Han X, Wong YS, Wong MH, Tam NFY (2008) Effects of anion species and concentration on the removal of Cr(VI) by a microalgal isolate Chlorella miniata. J Hazard Mater 158(2–3):615–620. doi:10.1016/j.jhazmat.2008.02.024
Haxo FT (1960) Comparative biochemistry of photoreactive systems. Academic Press, MB Allen New York, pp 339–360
Holman H-YN, Perry DL, Martin MC, Lamble GM, McKinney WR, Hunter-Cevera JC (1999) Real-time characterization of biogeochemical reduction of Cr(VI) on basalt surfaces by SR-FTIR imaging. Geomicrobiol J 16(4):307–324
ISO Wq (1994) Determination of chromium (VI)—spectrometric method using 1,5-diphenylcarbazide
Kadirvelu K, Namasivayam C (2000) Agricutural by-product as metal adsorbent: sorption of lead(II) from aqueous solution onto Coirpith carbon. Environ Technol 21(10):1091–1097
Kratochvil D, Pimentel P, Volesky B (1998) Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ Sci Technol 32(18):2693–2698. doi:10.1021/es971073u
Laxman RS, More S (2002) Reduction of hexavalent chromium by Streptomyces griseus. Miner Eng 15(11):831–837. doi:10.1016/s0892-6875(02)00128-0
Lin Z, Wu J, Xue R, Yang Y (2005) Spectroscopic characterization of Au3 + biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim Acta Part A Mol Biomol Spectrosc 61(4):761–765. doi:10.1016/j.saa.2004.03.029
Sharma M, Kaushik A, Kaushik CP (2011) Int Biodeterior Biodegradation 65(4):656–663
Srinath T, Garg SK, Ramteke PW (2002) Chromium (VI) accumulation by Bacillus circulans: effect of growth conditions. Indian J Microbiol 142(2):141–146
Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wunschiers R, Lindblad P (2002) Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev 66(1):1–20. doi:10.1128/mmbr.66.1.1-20.2002
Vankar PS, Srivastava J (2008) Comparative study of total phenol, flavonoid contents and antioxidant activity in Canna indica and Hibiscus rosa sinensis: prospective natural food dyes. Int J Food Eng 4(3)
Zhou M, Liu Y, Zeng G, Li X, Xu W, Fan T (2007) Kinetic and equilibrium studies of Cr(VI) biosorption by dead. Bacillus licheniformis biomass. World J Microbiol Biotechnol 23(1):43–48. doi:10.1007/s11274-006-9191-8
Acknowledgments
The authors express their sincere thanks to Ministry of Environment and Forest (MOEF), Govt. of India, New Delhi for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shukla, D., Vankar, P.S. & Srivastava, S.K. Bioremediation of hexavalent chromium by a cyanobacterial mat. Appl Water Sci 2, 245–251 (2012). https://doi.org/10.1007/s13201-012-0044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0044-3