Abstract
Fluorinated TiO2 composites were prepared by the sol–gel method. The photocatalytic activities of the composites were evaluated with bromophenol blue (BPB). Composites which contained carbon nanotubes showed the highest photodegradation (98%) of BPB at 20 min as compared to pure TiO2. Photodegradation by-products of BPB such as Br− and [SO4]2− ions were monitored with ion chromatography. All catalysts were characterized with microscopic techniques, including transmission electron microscopy and scanning electron microscopy which were both equipped with an energy dispersive X-ray spectrophotometer. The polymorph of TiO2 was verified by the use of Raman spectroscopy and powder X-ray diffraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wastewaters containing organic pollutants, such as dyes from textile industries are of concern because of their hazardous effects on the environment (Smith et al. 2009). Their presence in water, even at low concentrations has been the driving force for improved water treatment techniques. An example of such dyes is bromophenol blue (BPB), the structure of which is shown in Fig. 1 (Masere and Pojman 1998). This compound has absorption characteristics for λmax of 589 and 435 nm at pH 4.6 and 3.0, respectively.
Titanium dioxide (TiO2) is the preferred photo-catalyst for water treatment, because of its low cost, high stability, and non-toxicity particularly for the mineralization of organic pollutants (Lensebigler et al. 1995; Fujishima and Honda 1972). However, the use of TiO2 is hampered by two primary factors. The first is the fast recombination rate of photo-generated electron/hole (e−/h+) pairs. The second is the large band gap (3.2 eV) which makes TiO2 incapable of utilizing visible light, that accounts for about 50% of solar energy (Lensebigler et al. 1995; Fujishima and Honda 1972; Ni et al. 2009).
Therefore, to improve the performance of TiO2 and enhance its photo-catalytic activity, several modifications have been suggested: such as noble metal loading, anion doping and dye sensitisation (Lensebigler et al. 1995; Ni et al. 2009). The use of metals as dopants has several drawbacks, including reduced thermal stability as well as the fact that metal centres act as recombination centres which allows for fast recombination of the produced electron/hole pairs (Lensebigler et al. 1995; Ni et al., 2009). All the mentioned drawbacks result in decreased photo-catalytic efficiency. The toxicity and instability of dye-sensitized TiO2 limits its application in photo-catalysis. However, anion-doped TiO2 [N, (Ashi et al. 2001, Kontos et al. 2011); F, (Minero et al. 2000a; Selli et al. 2011; Chen et al. 2009); S, (Ohno et al., 2004); C, (Sakthivel and Kisch 2003; Matos et al. 2010)] have shown reduced effective band gaps and improved photocatalytic activity.
Mrowetz and Selli (2005) have experimentally demonstrated that fluorination of TiO2, enhanced photo-catalytic activity by generating hydroxyl radicals. The increased of free hydroxyl radicals results in an increase in the rate of degradation of organic pollutants. Also, the recombination of holes and electrons is minimized with fluorination, thus further enhancing photo-catalytic activity of TiO2.
Composites containing CNTs have the potential to be used in various applications as they exhibit synergistic effects between the metal oxide and carbon phase (Eder and Windle 2008). CNTs/TiO2 composite materials have also been shown to increase the rate of photocatalytic oxidation of organic pollutants. In this paper, we present the synthesis and characterization of a composite of fluoride-doped TiO2 with CNTs and the evaluation of its photoactivity with BPB.
Experimental
Catalyst preparation
One step fluorination of TiO2 with CNTs was successfully achieved via a sol–gel process. Commercial multi-walled CNTs (400 mg) with a diameter range of 110–170 nm and 5–9 μm length were refluxed in nitric acid (50 mL) for 16 h. This was done to introduce functional groups on the surface of CNTs. The functionalized CNTs (0.2% m/m) were sonicated in 10 mL n-butanol for 15 min. The dispersed CNTs were mixed with titanium butoxide (10 mL) and n-butanol (42 mL). The mixture was stirred continuously for 30 min and followed by the addition of formic acid (11 mL) (Zhu et al. 2005). Once a grey precipitate was formed.
NH4F (10 mL, 20% m/m) prepared in 20 mM HF was added. The pH was maintained at 2, to allow for the exchange of hydroxyl ion with fluoride ions. The precipitate was stirred for 2 h and aged for another 2 h. After the precipitate had been washed with n-butanol and water, it was dried for 2 h at 80°C to remove most of the solvent. The dried sample was ground to fine powder and calcined in the furnace at a ramp rate of 10°C/min to a maximum temperature of 400°C in air for 4 h. Pure TiO2 and CNTs-TiO2 were also prepared, following the same method.
Characterization
X-ray scattering patterns were scanned by analyzing the powdered samples using a Siemens Seifert 3000TT diffractometer using Cu Kα (0.1540 nm) monochromatic beam. The power settings of the source were 40 kV and 40 mA. Diffraction patterns were taken with a scan rate of 5°/min over the range 20° ≤ 2θ ≤ 60°. The average crystal sizes, d, of anatase were determined by the Scherrer equation for a given phase θ, at an X-ray wavelength λ 0.1540 nm and FWHM (full width at half maximum, β).
Scanning electron microscopy (SEM) images were taken at an accelerated voltage of 2 kV using a Nova NanoSEM 600 instrument (FEI Co., The Netherlands) equipped with an X-ray detector for energy dispersive X-ray analysis (EDX) operated at 15 kV. Transmission electron microscopy (TEM) images were captured using JEOL-3010 microscope at an accelerated voltage of 200 kV. Samples were dispersed in methanol and drop-dried on a copper grid. Raman spectra were collected with a Horiba Jobin–Yvon spectrometer at an ambient temperature using an (Ar+) excitation source of 514.5 nm, with a spectral resolution of 2 cm−1. The laser power at the sample was about 3.0 mW. The laser beam was focused onto the sample by a 100× magnification objective lens of an Olympus microscope.
Photocatalytic measurements
Bromophenol blue was used as a model pollutant to evaluate the photocatalytic activity of the prepared catalysts. Each of the four (TiO2, CNTs-TiO2, TiO2-F and CNTs-TiO2-F) catalysts (0.1 g) was suspended in a solution of BPB (100 mL, 10 mgL−1). Prior to the irradiation, the reaction vessel was magnetically stirred for 30 min to ensure an adsorption/desorption equilibrium in the dark. The photodegradation was initiated by a UV lamp (55 Watts) at 366 nm and the cabin temperature was measured to be at 45 ± 5°C for all devices. Sample aliquots (5 mL) were taken at regular intervals (20 min) and filtered through 0.45 μm Teflon membranes.
The degradation of BPB was monitored by measuring the absorbance at λ = 435 nm as a function of irradiation time with a UV–vis spectrophotometer (Shimadzu UV-2450). The resulting Br− and [SO4]2− ions were measured with an ion chromatograph (IC, Dionex-ICS 2000) that was equipped with a Dionex Ion Pac AS18 (2 × 250 mm) column and a conductivity. The eluent solution was 30 mM KOH. In this study, the photocatalytic degradation was calculated as the following formulae:
where C0 and C are the initial and final concentrations of BPB at t = 0 and t.
Results and discussion
Figure 2 shows the XRD patterns of TiO2 and CNTs-TiO2, containing 0.2% m/m of CNTs. Intense peaks at 2θ = 25.5, 38.2, 48.6, 54.0 and 55.3°, corresponding to crystal planes of TiO2 anatase (101), (004), (200), 108) and (211), respectively, using JCPDS card 04047 (Diebold 2002). All of the patterns and planes can be exclusively assigned to anatase, a polymorph of TiO2, which is reported to be photocatalytically superior to the other forms of TiO2 (Smith et al. 2009). By applying the Scherrer equation for (101) reflection, the average size of TiO2 was calculated to be 20 nm. The Raman spectra shown in Fig. 3 also assign the 144 cm−1 (very strong Eg), 397 cm−1 (B1g), 516 cm−1 (A1g) and 639 cm−1 (Eg) bands to anatase as confirmed by XRD. Raman analysis of the CNTs-TiO2 composite indicates the presence of the G band at 1,611 cm−1 resulting from CNTs, indicating the crystalline nature of CNTs.
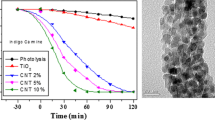
TEM and SEM images in Fig. 4 reveal that the TiO2 particles were partly agglomerated and yet their average size distribution was uniform. Both microscopic techniques estimated the average particle size to be 16 nm, similar to XRD estimates. An elemental composition of the composite was elucidated with EDX and confirmed the presence of carbon, fluorine, titanium and oxygen.
Rapid photocatalytic degradation of BPB by CNTs-TiO2-F was observed with a percentage removal of 98% of BPB at 20 min of illumination. Complete mineralization of BPB was maintained throughout the irradiation time. The as-prepared TiO2 showed the least photoactivity of all four catalysts, managing to degrade up to 85% of BPB at 140 min. CNTs-TiO2 and TiO2-F, degraded BPB up to 94% in140 min irradiation time. The reaction kinetics all show that the reactions followed a pseudo first order, increasing from 0.0178 min−1 (TiO2) to 0.0673 min−1 (CNTs-TiO2-F) (Fig. 5).
The degradation efficiency of the CNTs-TiO2-F was the highest, because CNTs are excellent electron transporters. Delocalization of π-bonds that form from unhybridized p-orbitals (Eder 2010), allows CNTs to be conducting; thus efficiently separating electrons from holes, as in Scheme 1 (Eder 2008). This can be attributed to the high percentage degradation of BPB due to the high surface area of CNTs that also enhances the adsorption of BPB to be degraded by TiO2.
Fluorinated TiO2 catalyst degraded BPB efficiently because of the excess production of free radicals as compared to pure TiO2. When TiO2 is fluorinated, the surface \( \left( { \equiv {\text{Ti}} \to {\text{F}}} \right) \) acts as an electron-trapping site, tightly holding the electrons (due to the high electronegativity of fluorine), thereby minimizing chances of recombination (Minero et al. 2000a, b; Mrowetz and Selli 2005). This then leads to increased photocatalytic activity, of TiO2-F with BPB. Furthermore, since fluoride ions have a high potential (3.6 V), valence band holes do not directly oxidize the surface of titanium, but directly react with water molecules, producing more OH· radicals as illustrated by (3) (Mrowetz and Selli 2005; Park and Choi 2004). An increase in hydroxyl radical production enhances the photocatalytic degradation of BPB. Preliminary experiments conducted in our laboratories with 2,2–diphenyl-1-picryhydrazyl (DPPH) also showed that more hydroxyl radicals were liberated with fluorinated TiO2 as compared to un-fluorinated catalyst.
To verify the degradation of BPB the liberation of Br− and [SO4]2− ions were measured with an IC. As illustrated, in Fig. 6, Br− ions were liberated at much higher amounts than the [SO4]2− ions for all four catalysts, confirming that all four bromine atoms were removed from BPB in the case of fluorinated TiO2.
The degradation mechanism of organic dyes from the use of TiO2 proceeds via the formation of e−/h+ pairs upon illumination with energy greater or equal to 3.2 eV (4). The generated holes may then react with water producing OH· radicals (5). These OH· radicals are powerful oxidizing agents which can then degrade the organic dye (6). However, the degradation of the dye can also be achieved via the direct reaction of the generated holes (7). In addition to the mentioned possible degradation pathways involving holes, the photogenerated electrons can either reduce the dye or can act with the electron acceptor to create a superoxide, O −2 thereby mineralizing the dye (8). (Lensebigler et al. 1995; Ni et al. 2009; Kudo and Miseki 2009):
Conclusion
TiO2 nanocomposites were successfully synthesised and characterized with microscopic (TEM and SEM) and spectroscopic (XRD and Raman) techniques. CNTs-TiO2-F had the highest photocatalytic activity of BPB, 98% removal in 20 min irradiation time, while prepared TiO2 showed the least activity of 85% in 120 min. The high photocatalytic activity of CNTs-TiO2-F can be attributed to the increase in production of OH· radicals as attested by previous studies. The efficiency of the composite is also enhanced by efficiently maximizing the separation of electrons from holes by CNTs, as they act as electron transporters. The liberation of Br- and [SO4]2− ions confirms the mineralization of the dye. Kinetics measurements revealed that the photodegradation followed a pseudo first order kinetics.
References
Ashi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Chen Y, Chen F, Zhang J (2009) Effect of surface fluorination on the photocatalytic and photoinduced hydrophilic properties of porous TiO2 films. Appl Surf Sci 255:6290–6296
Diebold U (2002) The surface of titanium dioxide. Surf Sci Rep 48:53–229
Eder D (2010) Carbon nanotube—inorganic hybrids. Chem Rev 110:1348–1385
Eder D, Windle AH (2008) Carbon-inorganic hybrid materials: the carbon nanotube/TiO2 interface. Adv Mater 20:1787–1793
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 72:108–114
Kontos AG, Pelaez M, Likodimos V, Vaenas N, Dionysiou DD, Falaras P (2011) Visible light induced wetting nanostructured N–F co-doped titania films. Photochem Photobiol Sci 10:350–354
Kudo A, Miseki Y (2009) Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev 38:253–278
Lensebigler AL, Lu G, Yates JT (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms and selected results. Chem Rev 95:735–758
Masere J, Pojman JA (1998) Free radical-scavenging dyes as indicators of frontal polymerization dynamics. J Chem Soc Faraday Trans 94:919–922
Matos J, Lopez EG, Palmisanol L, Garcia A, Marci G (2010) Influence of activated varbon in TiO2 and ZnO mediated photo-assisted degradation of 2-propanol in gas-solid regime. Appl Catal B: Environ 99:170–180
Minero C, Mariella G, Maurino V, Pelizzetti E (2000a) Photocatalytic transformation of organic compounds in the presence of inorganic anion1. Hydroxyl-mediated and direct electron transfer reactions of phenol on titanium fluoride system. Langmuir 16:2632–2641
Minero C, Mariella G, Maurino V, Vione D, Pelizzetti E (2000b) Photocatalytic transformation of organic compounds in the presence of inorganic anion2. Competitive reactions of phenol and alcohols on a titanium dioxide fluoride system. Langmuir 16:8964–8972
Mrowetz M, Selli E (2005) Enhanced photocatalytic formation of hydroxyl radicals on fluorinated TiO2. Phys Chem Chem Phys 7:1100–1102
Ni M, Leung MKH, Leung DYC, Sumathy K (2009) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401–425
Ohno T, Akiyoshi M, Umebayashi K, Asai T, Mitsui T, Matsumura M (2004) Preparation of S-doped TiO2 photocatalyst and their photocatalytic activities under visible light. Appl catal A 265:115–121
Park H, Choi W (2004) Effects of TiO surface fluorination on photocatalytic reactions and photoelectrochemical behaviours. Phys Chem B 108:4086–4093
Sakthivel S, Kisch H (2003) Daylight photocatalysis by carbon-modified titanium dioxide. Angew Chem Int Ed 42:4908–4911
Selli E, Dozzi MV, Livraghi S, Giamello E (2011) Photocatalytic activity of S- and F- doped TiO2 in formic acid mineralization. Photochem Photobiol Sci 10:343–349
Smith YR, Kar A, Subramanian VR (2009) Investigation of physicochemical parameters that influence photocatalytic degradation of methyl orange over TiO2 nanotubes. Ind Eng Chem Res 48:10268–10276
Zhu J, Zhang J, Chen F, Iino K, Anpo M (2005) High activity TiO2 photocatalyst prepared by a modified sol-gel method: Characterization and their photocatalytic activity for the degradation of XRG and X-GL. Catalysis 35:261–268
Acknowledgments
Funding from the University of Johannesburg, ESKOM’s Tertiary Support Program (TESP) and the Department of Science and Technology, Centre of Excellence in Strong Materials (DST/CoESM) is appreciated. LN Dlamini thanks Dr. Sharon Moeno for her assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dlamini, L.N., Krause, R.W., Kulkarni, G.U. et al. Photodegradation of bromophenol blue with fluorinated TiO2 composite. Appl Water Sci 1, 19–24 (2011). https://doi.org/10.1007/s13201-011-0004-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-011-0004-3