Abstract
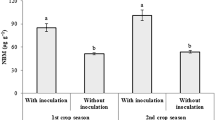
Cowpea is one of the most important food legume crops in Burkina Faso. It is able to associate with arbuscular mycorrhizal fungi (AMF) and rhizobia. This dual symbiosis improves nitrogen and phosphorus nutrient uptake in cowpea. As the application of exotic inoculants frequently lacks positive responses in field experiments, this study set out to select well-adapted native symbiotic rhizobial and AMF strains. Soil samples were collected from six study sites in three different climatic zones of Burkina Faso to investigate their native symbiotic strains. Soil-extraction of native spores led to the identification of four AMF genera (Scutellospora, Gigaspora, Glomus and Entrophospora) by morpho-anatomical characterization. The two most effective cowpea fungal strains were selected after spore isolation from field-collected soils, multiplication on maize roots and inoculation on cowpea seedlings in a greenhouse experiment. Cowpea-nodulating rhizobial strains were trapped in the greenhouse by planting cowpea seeds in collected soil samples and the strains were characterized using molecular methods. This characterization led to the rhizobial isolates being classified in four clusters on the phylogenetic tree (using the Maximum-Likelihood Phylogenies method). All strains belonged to the Bradyrhizobium genus and most of them were included in the B. japonicum branch. Some groups were clearly distinct species already identified and may be new species. The two most effective strains for cowpea yield improvement in the field were selected after cowpea inoculation in a greenhouse experiment. The inoculation design in the field experiment consisted of four single inoculation treatments, either rhizobial or mycorrhizal, along with four dual inoculations, one treatment with chemical fertilizers, and one uninoculated control. The results showed that cowpea productivity was significatively improved by dual inoculation with native rhizobial and mycorrhizal strains, reaching the same level as the application of commonly used chemical fertilizers [Nitrogen, Phosphorus and Potassium fertilizers (NPK)]. In addition, dual inoculation resulted in the highest iron content in cowpea leaves.





Similar content being viewed by others
References
Aboubacar K, Ousmane ZM, Amadou HI, Issaka S, Zouberou AM (2013) Effet de la co-inoculation du rhizobium et de mycorhizes sur les performances agronomiques du niébé [Vigna unguiculata (L.) Walp.] au Niger. J Appl Biosci 72:5846–5854
Ahmad M, Zahir ZA, Khalid M, Nazli F, Arshad M (2013) Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer's fields. Plant Physiol Biochem : PPB / Societe francaise de physiologie vegetale 63:170–176. doi:10.1016/j.plaphy.2012.11.024
Alguacil MM, Lozano Z, Campoy MJ, Roldán A (2010) Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biol Biochem 42:1114–1122. doi:10.1016/j.soilbio.2010.03.012
Araujo J, Díaz-Alcántara CA, Velázquez E, Urbano B, González-Andrés F (2015) Bradyrhizobium yuanmingense related strains form nitrogen-fixing symbiosis with Cajanus cajan L. in Dominican Republic and are efficient biofertilizers to replace N fertilization. Sci Hortic 192:421–428. doi:10.1016/j.scienta.2015.06.009
Arumugam R, Rajasekaran S, Nagarajan SM (2010) Response of arbuscular mycorrhizal fungi and rhizobium inoculation on growth and chlorophyll content of Vigna unguiculata (L) Walp Var. Pusa 151. J Appl Sci Environ Manag 14:113–115
Aserse AA, Rasanen LA, Aseffa F, Hailemariam A, Lindstrom K (2012) Phylogenetically diverse groups of Bradyrhizobium isolated from nodules of Crotalaria spp., Indigofera spp., Erythrina brucei and Glycine max growing in Ethiopia. Mol Phylogenet Evol 65:595–609. doi:10.1016/j.ympev.2012.07.008
Azarias Guimaraes A, Duque Jaramillo PM, Simao Abrahao Nobrega R, Florentino LA, Barroso Silva K, de Souza Moreira FM (2012) Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Appl Environ Microbiol 78:6726–6733. doi:10.1128/AEM.01303-12
Bâ AM, Dalpé Y, Guissou T (1996) Les glomales D'acacia holosericea et D'acacia mangium. Bois et Forêts des Tropiques 250:5–18
Beck DP, Materon LA, Afandi F (1993). Practical Rhizobium-legume technology manual. vol 19. Aleppo: International Center for Agricultural Research in the Dry Areas (ICARDA)
Bressani R (1985). Nutritive value of cowpea. In: Cowpea research, production and utilization, Singh and Rachies (eds) John Wiley and Sons, New York, p. 353–359
Dixon RK, Garg VK, Rao MV (1993) Inoculation of Leucaena and Prosopis seedlings with Glomus and Rhizobium species in saline soil: rhizosphere relations and seedling growth. Arid Land Res Manag 7:133–144. doi:10.1080/15324989309381343
Duponnois R, Plenchette C, Thioulouse J, Cadet P (2001) The mycorrhizal soil infectivity and arbuscular mycorrhizal fungal spore communities in soils of different aged fallows in Senegal. Appl Soil Ecol 17:239–251. doi:10.1016/s0929-1393(01)00132-9
Duponnois R, Colombet A, Hien V, Thioulouse J (2005) The mycorrhizal fungus Glomus intraradices and rock phosphate amendment influence plant growth and microbial activity in the rhizosphere of Acacia holosericea. Soil Biol Biochem 37:1460–1468. doi:10.1016/j.soilbio.2004.09.016
Fitter AH (2006) What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol 172:3–6. doi:10.1111/j.1469-8137.2006.01861.x
Franco AA, Munns DN (1982) Acidity and aluminum restraints on nodulation, nitrogen fixation, and growth of Phaseolus vulgaris in solution culture. Soil Sci Soc Am J 46:296–301. doi:10.2136/sssaj1982.03615995004600020016x
Galloway R, Dusch E, Elder L, Achadi E, Grajeda R, Hurtado E, Favin M, Kanani S, Marsaban J, Meda N, Moore KM, Morison L, Raina N, Rajaratnam J, Rodriquez J, Stephen C (2002) Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc Sci Med 55:529–544. doi:10.1016/S0277-9536(01)00185-X
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244. doi:10.1016/s0007-1536(63)80079-0
Gibson AH (1963) Physical environment and symbiotic nitrogen fixation. Aust J Biol Sci 16:28–42. doi:10.1071/BI9630028
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi:10.1093/molbev/msp259
Graham-Weiss L, Bennett ML, Paau AS (1987) Production of bacterial inoculants by direct fermentation on nutrient-supplemented vermiculite. Appl Environ Microbiol 53:2138–2141
Haro H (2011). Effet d’inoculums de champignons mycorhiziens arbusculaires sur la productivité du niébé Vigna unguiculata (L.) Walp. Diplôme d’Etudes Approfondies (DEA) en Biotechnologie Microbienne et Cellulaire, Université de Ouagadougou, Burkina Faso
Haro H, Sanon KB, Diop I, Kane A, Dianda M, Houngnandan P, Neyra M, Traoré A (2012) Réponse à l'inoculation mycorhizienne de quatre variétés de niébé [Vigna unguiculata (L.) Walp.] cultivées au Burkina Faso et au Sénégal. Int J Biol Chem Sci 6:2097–2112. doi:10.4314/ijbcs.v6i5.18
Haro H, Sanon KB, Krasova-Wade T, Kane A, N’Doye I, Traoré AS (2015) Réponse à la double inoculation mycorhizienne et rhizobienne du niébé (variété, KVX396-4-5-2D) cultivé au Burkina Faso. Int J Biol Chem Sci 9:1485–1493. doi:10.4314/ijbcs.v9i3.31
Hatimi A, Bani-Aameur F, Oihabi A (2001) Caractérisation de souches de Rhizobiums autochtones des dunes: effet sur la croissance et la nutrition azotée D'acacia cyanophylla Lindl. Acta Bot Gall 148:191–199. doi:10.1080/12538078.2001.10515887
Havugimana E, Bhople BS, Byiringiro E, Mugabo JP (2015) Role of Dual Inoculation of Rhizobium and Arbuscular Mycorrhizal (AM) Fungi on Pulse Crops Production Walailak. J Sci Techno (WJST) 13:1–7. doi:10.14456/WJST.2016.1
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop Res 65:151–164. doi:10.1016/s0378-4290(99)00084-2
Iqbal A, Khalil IA, Ateeq N, Sayyar Khan M (2006) Nutritional quality of important food legumes. Food Chem 97:331–335. doi:10.1016/j.foodchem.2005.05.011
Khaled LB, Gomez AM, Quarraqi El M, Oihabi A (2003) Réponses physiologiques et biochimiques du trèfle (Trifolium alexandinum. L.) à la double association mycorhizes rhizobiums sous une contrainte saline. Agronomie 23:571–580. doi:10.1051/agro:2003037
Kim BH, Ramanan R, Cho DH, Oh HM, Kim HS (2014) Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 69:95–105. doi:10.1016/j.biombioe.2014.07.015
Koske R, Tessier B (1983) A convenient, permanent slide mounting medium. Mycological Society of America Newsletter 34:59
Leye EHM, Ndiaye M, Ndiaye F, Diallo B, Sarr AS, DIOUF M, Diop T (2009) Effet de la mycorhization sur la croissance et le développement de Jatropha curcas L. Rev Energ Renouv 12:269–278
Leye EHM, Ndiaye M, Diouf M, Diop T (2015) Etude Comparative de L’effet de Souches de Champignons Mycorhiziens Arbusculaires sur la Croissance et la Nutrition Minérale du Sésame Cultivé au Sénégal. Afr Crop Sci J 23:211–219
Marschner H, Dell B (1994) Nutrient-uptake in mycorrhizal Symbiosis. Plant Soil 159:89–102. doi:10.1007/BF00000098
Menna P, Hungria M (2011) Phylogeny of nodulation and nitrogen-fixation genes in Bradyrhizobium: supporting evidence for the theory of monophyletic origin, and spread and maintenance by both horizontal and vertical transfer. Int J Syst Evol Microbiol 61:3052–3067. doi:10.1099/ijs.0.028803-0
Mikola P (1987) Mycorrhizae under tropical stresses. Angew Bot 61:15–23
Morris ML (2007) Fertilizer use in African agriculture: lessons learned and good practice guidelines. World Bank Publications, Washington, DC
Morton JB (1988) Taxonomy of VA mycorrhizal fungi: classification, nomenclature, and identification. Mycotaxon 32:267–324
Musoko M, Last FT, Mason PA (1994) Populations of spores of vesicular-arbuscular mycorrhizal fungi in undisturbed soils of secondary semideciduous moist tropical forest in Cameroon. For Ecol Manag 63:359–377. doi:10.1016/0378-1127(94)90117-1
Nkouannessi M (2005) The genetic, morphological and physiological evaluation of african cowpea genotypes. Magister Scientiae Agriculturae, University of the Free State, Bloemfontain.
Ouedraogo JT, Drabo I, Tignegre JB, Dabire C, Sereme P, Konate G (2003) Fiches techniques du niébé variétés KVX 396–4-5-2D, KVX 61–1, KVX 745-11P, Gorom local. INERA, Burkina Faso
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Plenchette C, Clermont-Dauphin C, Meynard JM, Fortin JA (2005) Managing arbuscular mycorrhizal fungi in cropping systems. Can J Plant Sci 85:31–40. doi:10.4141/p03-159
Ponsonnet C, Nesme X (1994) Identification of Agrobacterium strains by PCR-RFLP analysis of pTi and chromosomal regions. Arch Microbiol 161:300–309. doi:10.1007/bf00303584
Redhead JF (1977) Endotrophic mycorrhizas in Nigeria: species of the Endogonaceae and their distribution. Trans Br Mycol Soc 69:275–280
Robson AD (1983) Mineral nutrition. In: WJ Broughton CP, Oxford (ed) Nitrogen fixation of legumes. p. 37–55
Sanchez PA (1977) Properties and Management of Soils in the tropics. Soil Sci 124:187
Sánchez AC, Gutiérrez RT, Santana RC, Urrutia AR, Fauvart M, Michiels J, Vanderleyden J (2014) Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Eur J Soil Biol 62:105–112. doi:10.1016/j.ejsobi.2014.03.004
Sanz-Saez A, Heath KD, Burke PV, Ainsworth EA (2015) Inoculation with an enhanced N -fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated [CO2]. Plant, Cell Environ 38:2589–2602. doi:10.1111/pce.12577
Shi G, Liu Y, Johnson NC, Olsson PA, Mao L, Cheng G, Jiang S, An L, Du G, Feng H (2014) Interactive influence of light intensity and soil fertility on root-associated arbuscular mycorrhizal fungi. Plant Soil 378:173–188. doi:10.1007/s11104-014-2022-z
Singh B, Chambliss O, Sharma B (1997) Recent advances in cowpea breeding. Advances in Cowpea Research, lnternational lnstitute of Tropical Agriculture, Ibadan, Nigeria, Japan International Research Center for Agricultural Sciences Tsukuba, lbaraki, Japan edn
Singleton PW, Abdelmagid HM, Tavares JW (1985) Effect of phosphorus on the effectiveness of strains of Rhizobium japonicum. Soil Sci Soc Am J 49:613–616
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358. doi:10.1111/j.1469-8137.2008.02753.x
Solaiman MZ, Abbott LK (2003) Phosphorus uptake by a community of arbuscular mycorrhizal fungi in jarrah forest. Plant Soil 248:313–320. doi:10.1023/a:1022372228545
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume-Rhizobium technology. Springer-Verlag, New York
Trouvelot A, Kough JL, Gianinazzi-Pearson V, Gianinazzi S (1986). Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle Mycorrhizae: Physiology and Genetics p 217–221
Verma JP, Yadav J, Tiwari KN, Kumar A (2013) Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng 51:282–286. doi:10.1016/j.ecoleng.2012.12.022
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. Blackwell Scientific Publication, Oxford - Edinburgh
Willems A, Coopman R, Gillis M (2001) Comparison of sequence analysis of 16S-23S rDNA spacer regions, AFLP analysis and DNA-DNA hybridizations in Bradyrhizobium. Int J Syst Evol Microbiol 51:623–632
Yadav J, Verma JP (2014) Effect of seed inoculation with indigenous Rhizobium and plant growth promoting rhizobacteria on nutrients uptake and yields of chickpea (Cicer arietinum L.) Eur J Soil Biol 63:70–77. doi:10.1016/j.ejsobi.2014.05.001
Zaidi A, Khan MS, Amil M (2003) Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinum L.) Eur J Agron 19:15–21. doi:10.1016/s1161-0301(02)00015-1
Acknowledgements
This study benefited from the support of Agence Universitaire de la Francophonie (AUF) and the Cultural and Cooperation Service of the French Embassy (SCAC) in Burkina Faso. The authors wish to thank Peter Biggins for reviewing the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Haro, H., Sanon, K.B., Le Roux, C. et al. Improvement of cowpea productivity by rhizobial and mycorrhizal inoculation in Burkina Faso. Symbiosis 74, 107–120 (2018). https://doi.org/10.1007/s13199-017-0478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-017-0478-3