Abstract
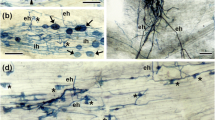
The role of ethylene on arbuscular mycorrhiza (AM) development remains unclear. We used tomato ethylene mutants in near isogenic genotypes to evaluate AM development and growth differences and whether changes in levels of plant defense-related transcripts in roots were correlated with those differences. The cultivar Micro-Tom (MT) and mutants overproducing ethylene (epinastic, epi) and insensitive to ethylene (Never-ripe, Nr) were inoculated with Glomus clarum. The epi mutants exhibited reduced intraradical colonization at all time-points and reduced frequencies of hyphopodia and arbuscules at 30 and 37 days after inoculation (DAI) compared to MT. At these times and 23 DAI, epi roots contained higher levels of mRNAs encoding Chi3 and Chi9 chitinases, Cu-Zn superoxide dismutase, catalase and a peroxidase than MT roots. In contrast, Nr showed higher intraradical colonization and frequencies of arbuscules, external hypha and hyphopodia but only at 23 DAI. At the preceding 16 DAI time point, levels of antioxidant enzyme mRNAs were higher while Chi3, Chi9 and GlucB mRNAs were lower than in MT roots. Our study provides evidence that specific stages of AM development were altered negatively in epi and positively in Nr. These effects on fungal growth are most likely due to altered ethylene signaling modulating the plant defense system.







Similar content being viewed by others
References
Aimé S, Cordier C, Alabouvette C, Olivain C (2008) Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol Mol Plant P 73:9–15
Azcón-Aguilar C, Rodrigues-Navarro DN, Barea JM (1981) Effects of ethrel on the formation and responses to VA mycorrhiza in Medicago and Triticum. Plant Soil 60:461–468
Balestrini R, Gómez-Ariza J, Lanfranco L, Bonfante P (2007) Laser Microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol Plant Microbe In 20:1055–1062
Barker SJ, Stummer B, Gao L, Dispain I, O’Connor PJ, Smith SE (1998) A mutant in Lycopersicon esculentum Mill. With highly reduced VA mycorrhizal colonization: isolation and preliminary characterization. Plant J 15:791–797
Barry CS, Fox EA, Yen H, Lee S, Ying T, Grierson D, Giovannoni J (2001) Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol 127:58–66
Besmer YL, Koide RT (1999) Effect of mycorrhizal colonization and phosphorus on ethylene production by snapdragon (Antirrhinum majus L.) flowers. Mycorrhiza 9:161–166
Blee KA, Anderson AJ (1996) Defense-related transcript accumulation in Phaseolus vulgaris L. colonized by the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith. Plant Physiol 110:675–688
Blilou I, Bueno P, Ocampo JA, Garcia-Garrido JM (2000) Induction of catalase and ascorbate peroxidase activities in tobacco roots inoculated with the arbuscular mycorrhizal Glomus mosseae. Mycol Res 194:722–725
Bouchez O, Huard C, Lorrain S, Roby D, Balagué C (2007) Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis lesion mimic mutant vad1. Plant Physiol 145:465–477
Bressano M, Curetti M, Giachero L, Gil SV, Cabello M, March G, Ducasse DA, Luna CM (2010) Mycorrhizal fungi symbiosis as a strategy against oxidative stress in soybean plants. J Plant Physiol 167:1622–1626
Campos ML, Carvalho RF, Benedito VA, Peres LEP (2010) Small and remarkable: the Micro-Tom model system as a tool to discover novel hormonal functions and interactions. Plant Signal Behav 5:50–54
Carvalho RF, Campos ML, Pino LE, Crestana SL, Zsögön A, Lima JE, Benedito VA, Peres LEP (2011) Convergence of developmental mutants into a single tomato model system: ‘Micro-Tom’ as an effective toolkit for plant development research. Plant Meth 7. doi:10.1186/1746-4811-7-18
Cruz AF, Ishii T, Kadoya K (2000) Effects of arbuscular mycorrhizal fungi on tree growth, leaf water potential, and levels of 1-aminocyclopropane-1carboxylic acid and ethylene in the roots of papaya under water-stress conditions. Mycorrhiza 10:121–123
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defense responses to infection. Nature 411:826–833
Fester T, Hause G (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15:373–379
Fiorilli V, Catoni M, Miozzi L, Novero M, Accotto GP, Lanfranco L (2009) Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol 184:975–987
Fujino DW, Burger DW, Yang SF, Bradford KJ (1988) Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. Cultivar VFN8). Plant Physiol 88:74–779
Fujino DW, Burger DW, Bradford KJ (1989) Ineffectiveness of ethylene biosynthetic and action inhibitors in phenotypically reverting the epinastic mutant of tomato (Lycopersicon esculentum Mill.). J Plant Growth Regul 8:53–61
Gao LL, Delp G, Smith SE (2001) Colonization patterns in a mycorrhiza-defective mutant tomato vary with different arbuscular-mycorrhizal fungi. New Phytol 151:477–491
Geil RD, Guinel FC (2002) Effects of elevated substrate-ethylene on colonization of leek (Allium porrum) by the arbuscular mycorrhizal fungus Glomus aggregatum. Can J Bot 80:114–119
Geil RD, Peterson RL, Guinel FC (2001) Morphological alterations of pea (Pisum sativum cv. Sparkle) arbuscular mycorrhizas as a result of exogenous ethylene treatment. Mycorrhiza 11:137–143
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U (2008) Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20:2989–3005
Ishii T, Shrestha YH, Matsumoto I, Kadoya K (1996) Effect of ethylene on the growth of vesicular-arbuscular mycorrhizal fungi and on the mycorrhizal formation of trifoliate orange roots. J Jpn Soc Hortic Sci 65:525–529
Kloppholz S, Kuhn H, Requena N (2011) A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol 21:1204–1209
Lambais MR, Mehdy MC (1993) Suppression of endochitinase, β-1,3-endoglucanase, and chalcone isomerase expression in bean vesicular-arbuscular mycorrhizal roots under different soil phosphate conditions. Mol Plant Microbe In 6:75–83
Lambais MR, Mehdy MC (1995) Differential expression of defense-related genes in arbuscular mycorrhiza. Can J Bot 73:533–540
Lambais MR, Mehdy MC (1998) Spatial distribution of chitinases and β-1,3-glucanase transcripts in bean arbuscular mycorrhizal roots under low and high soil phosphate conditions. New Phytol 140:33–42
Lambais MR, Rios-Ruiz WF, Andrade RM (2003) Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. New Phytol 160:421–428
Liu J, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ (2007) Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J 50:529–544
Lorrain S, Lin B, Auriac MC, Kroj T, Saindrenan P, Nicole M, Balague C, Roby D (2004) Vascular associated death1, a novel GRAM domain-containing protein, is a regulator of cell death and defense responses in vascular tissues. Plant Cell 16:2217–2232
Lund ST, Stall RE, Klee HJ (1998) Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10:371–382
McArthur DAJ, Knowles NR (1992) Resistance responses of potato to vesicular-arbuscular mycorrhizal fungi under varying abiotic phosphorus levels. Plant Physiol 100:341–351
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjärvi J (2002) Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 130:1918–1926
Morandi D (1989) Effect of xenobiotics on endomycorrhizal infection and isoflavonoid accumulation in soybean roots. Plant Physiol Bioch 27:697–701
Mukherjee A, Ané JM (2011) Germinating spore exudates from arbuscular mycorrhizal fungi: molecular and developmental responses in plants and their regulation by ethylene. Mol Plant Microbe In 24:260–270
Ortu G, Balestrini R, Pereira PA, Becker JD, Küster H, Bonfante P (2012) Plant genes related to gibberellin biosynthesis and signaling are differentially regulated during the early stages of AM fungal interactions. Mol Plant 5:951–954
Osorio S, Alba R, Damasceno CMB, Lopez-Casado G, Lohse M, Zanor MI, Tohge T, Usadel B, Rose JKC, Fei Z, Giovannoni JJ, Fernie AR (2011) Systems biology of tomato fruit development: combined transcript, protein and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol 157:405–425
Overmyer K, Brosché M, Kangasjarvi J (2003) Reative oxygen species and hormonal control of cell death. Trends Plant Sci 8:335–342
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Rev 6:763–775
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2001–2007
Riedel T, Groten K, Baldwin T (2008) Symbiosis between Nicotiana attenuate and Glomus intraradices: ethylene plays a role, jasmonic acid does not. Plant Cell Environ 31:1203–1213
Salzer P, Corbière H, Boller T (1999) Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208:319–325
Sarruge JR (1975) Soluções nutritivas. Summa Phytopathologica 1:231–2331
Schüssler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, Cambridge
Spanu P, Boller T, Ludwig A, Wiemken A, Faccio A, Bonfante-Fasolo P (1989) Chitinase in roots of mycorrhizal Allium porrum: regulation and localization. Planta 177:447–455
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Tejeda-Sartorius M, de la Vega OM, Délano-Frier JP (2008) Jasmonic acid influences mycorrhizal colonization in tomato plants by modifying the expression of genes involved in carbohydrate partitioning. Physiol Plantarum 133:339–353
Torres de Los Santos R, Vierheilig H, Ocampo JA, Garrido JMG (2011) Altered pattern of arbuscular mycorrhizal formation in tomato ethylene mutants. Plant Signal Behav 6:755–758
Tyburski J, Dunajska K, Mazurek P, Piotrowska B, Tretyn A (2009) Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol Plant 31:249–260
Vierheilig H, Alt M, Mohr U, Boller T, Wiemken A (1994) Ethylene biosynthesis and activities of chitinase and beta-1,3-glucanase in the roots of host and non host plants of vesicular–arbuscular mycorrhizal fungi after inoculation with Glomus mosseae. J Plant Physiol 143:337–343
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microb 64:5004–5007
Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270:1807–1809
Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliate) seedlings subjected to water stress. J Plant Physiol 163:1101–1110
Zsögön A, Lambais MR, Benedito VA, Figueira AVO, Peres LEP (2008) Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Sci Agric 65:259–267
Acknowledgments
GGMF was supported by FAPESP (São Paulo, Brazil). MRL and LEPP were supported by CNPq (Brasília, Brazil). This project was partially supported by CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fracetto, G.G.M., Peres, L.E.P., Mehdy, M.C. et al. Tomato ethylene mutants exhibit differences in arbuscular mycorrhiza development and levels of plant defense-related transcripts. Symbiosis 60, 155–167 (2013). https://doi.org/10.1007/s13199-013-0251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-013-0251-1