Abstract
Introduction
High-dose insulin (HDI) therapy has been used successfully for beta-blocker toxicity, but needs further study when hypotension persists despite HDI. The objective was to develop a model of propranolol toxicity with persistent hypotension despite HDI and to develop means to measure cerebral oxygen tension (PbrO2).
Methods
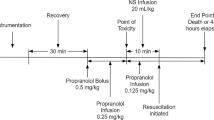
Eight anesthetized Yorkshire pigs were instrumented with a tracheostomy, Swan–Ganz catheter, arterial catheter, and intra-cerebral pressure and oxygen monitor. Intravenous propranolol was given until the initial point of toxicity (POT); 25% reduction from baseline mean arterial pressure (MAP) × heart rate (HR). At the initial POT, normal saline (NS) bolus and infusion along with HDI infusion were started. The propranolol infusion was titrated up slowly to induce hypotension. Group 2 pigs received a norepinephrine (NE) infusion after a secondary POT defined as a MAP < 50 mmHg. NE was titrated to maintain subsequent MAPs > 50 mmHg. Cardiac output, HR, MAP, PbrO2, and intracranial pressure were then recorded every 5 min until death or 4 h. Systemic vascular resistance, potassium, and glucose were also measured. Surviving pigs were euthanized.
Results
Two pigs received unique doses for protocol development. One pig developed a tachyarrhythmia prior to protocol, one failed to reach secondary POT, leaving 2 pigs in each group reaching secondary POT. The range of PbrO2 recordings for group 1 was 12.7–48.5 mmHg and 9.2–26.2 mmHg for group 2.
Conclusion
We report a pilot study swine model of propranolol toxicity with hypotension despite HDI, in which physiologic measures including PbrO2 are achieved. Our toxicity model can be used in the future, and the hemodynamic and brain monitoring model may prove important for subsequent research in various contexts.




Similar content being viewed by others
References
Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol. 2017;55:1072–252.
Holger JS, Stellpflug SJ, Cole JB, Harris CR, Engebretsen KM. High-dose insulin: a consecutive case series in toxin-induced cardiogenic shock. Clin Toxicol. 2011;49(7):653–8.
Holger JS, Engebretsen KM, Fritzlar SJ, Patten LC, Harris CR, Flottemesch TJ. Insulin versus vasopressin and epinephrine to treat beta-blocker toxicity. Clin Toxicol. 2007;45(4):396–401.
Manley GT, Pitts LH, Morabito D, Doyle CA, Gibson J, Gimbel M, et al. Brain tissue oxygenation during hemorrhagic shock, resuscitation, and alterations in ventilation. J Trauma. 1999;46(2):261–7.
Corporation IL. Integra® Licox® tunneling brain tissue oxygen and temperature monitoring kit 2010–2014 [cited 2014 Nov 20]. Available from: http://www.integralife.com/index.aspx?redir=detailproduct&Product=342&ProductName=Integra%AE Licox%AE Tunneling Brain Tissue Oxygen and Temperature Monitoring Kit&ProductLineName=Brain Tissue O2 Monitoring&ProductLineID=10&PA=neurosurgeon.
Cole JB, Stellpflug SJ, Ellsworth H, Anderson CP, Adams AB, Engebretsen KM, et al. A blinded, randomized, controlled trial of three doses of high-dose insulin in poison-induced cardiogenic shock. Clin Toxicol. 2013;51(4):201–7.
Holger JS, Engebretsen KM, Obetz CL, Kleven TL, Harris CR. A comparison of vasopressin and glucagon in beta-blocker induced toxicity. Clin Toxicol. 2006;44(1):45–51.
Lagerkranser M, Stange K, Sollevi A. Effects of propofol on cerebral blood flow, metabolism, and cerebral autoregulation in the anesthetized pig. J Neurosurg Anesthesiol. 1997;9(2):188–93.
Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183–238.
Bardt TF, Unterberg AW, Hartl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153–6.
Hemphill JC 3rd, Morabito D, Farrant M, Manley GT. Brain tissue oxygen monitoring in intracerebral hemorrhage. Neurocrit Care. 2005;3(3):260–70.
Metzger A, Rees J, Kwon Y, et al. Intrathoracic pressure regularion improves cerebral perfusion and cerebral blood flow in a porcine model of brain injury. Shock. 2017;44:96–102.
Kurita T, Kawashima S, Morita K, Nakajima Y. Use of a short acting B1 blocker during endotoxemia may reduce cerebral tissue oxygenation if hemodynamics are depressed by a decrease in heart rate. Shock. 2017;47:765–71.
Vogt N, Herden C, Roeb E, Roderfeld M, Eschbach D, Steinfeldt T, et al. Cerebral alterations following experimental multiple trauma and hemorrhagic shock. Shock. 2018;49:164–73.
Acknowledgments
In memory of Kristen Engebretsen PharmD, DABAT, a great clinician, colleague, and dedicated researcher who passed away before completion of this manuscript.
Source of Funding
This study was funded by a HealthPartners Discovery Grant, a grant completely internal to the investigators’ institution; no outside funding obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our study was approved and monitored independently by our institutional review board and animal care and use committee.
Conflicts of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orozco, B.S., Engebretsen, K.M., Holger, J.S. et al. A Swine Model of Severe Propranolol Toxicity Permitting Direct Measurement of Brain Tissue Oxygenation. J. Med. Toxicol. 15, 178–183 (2019). https://doi.org/10.1007/s13181-019-00707-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-019-00707-0