Abstract
Severe organophosphate compound (OPC) poisoning is an important clinical problem in many countries of the world. Unfortunately, little clinical research has been performed and little evidence exists with which to determine the best therapy. A study was therefore undertaken to determine the optimal dosing regimen for atropine in the treatment of OPC poisoning. An open-label randomized clinical trial was conducted in Chittagong Medical College Hospital, Chittagong, Bangladesh, on 156 hospitalized individuals with OPC poisoning from June to September 2006. The aim was to compare the efficacy and safety of conventional bolus doses with individualized incremental doses of atropine for atropinization followed by continuous atropine infusion for management of OPC poisoning. Inclusion criteria were patients with a clear history of OPC poisoning with clear clinical signs of toxicity, i.e. features of cholinergic crisis. The patients were observed for at least 96 h. Immediate outcome and complications were recorded. Out of 156 patients, 81 patients received conventional bolus dose atropine (group A) and 75 patients received rapidly incremental doses of atropine followed by infusion (group B). The mortality in group ‘A’ was 22.5% (18/80) and in group ‘B’ 8% (6/75) (p < 0.05). The mean duration of atropinization in group ‘A’ was 151.74 min compared to 23.90 min for group ‘B’ (p < 0.001). More patients in group A experienced atropine toxicity than in group ‘B’ (28.4% versus 12.0%, p < 0.05); intermediate syndrome was more common in group ‘A’ than in group ‘B’ (13.6% versus 4%, p < 0.05), and respiratory support was required more often for patients in group ‘A’ than in group ‘B’ (24.7% versus 8%, p < 0.05). Rapid incremental dose atropinization followed by atropine infusion reduces mortality and morbidity from OPC poisoning and shortens the length of hospital stay and recovery. Incremental atropine and infusion should become the treatment of choice for OPC poisoning. Given the paucity of existing evidence, further clinical studies should be performed to determine the optimal dosing regimen of atropine that most rapidly and safely achieves atropinization in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poisoning is a common emergency health problem worldwide, particularly in developing countries. In Bangladesh, poisoning is a major cause of death and hospital studies indicate that organophosphate compounds (OPC) account for the majority of fatal poisonings and morbidity due to poisoning there [1].
The case fatality rate for self-poisoning in the developing world is commonly greater than 20%, but for OPC it can be as high as 46% according to one review of studies from 96 hospitals [2].
The problem is compounded by a lack of research evidence on which to base treatment protocols, despite the large number of OPC poisoning cases occurring worldwide every year [3, 4]. Rapid and effective stabilization and treatment of OPC-poisoned patients in a hospital has great potential to reduce the number of early deaths, improve the short-term prognosis for surviving patients and reduce the number and severity of long-term sequelae. There is no consensus regarding diagnosis, grading of severity or management of OPC poisoning. OPC poisoning results from the action of OPC on acetylcholinesterase whose action it blocks, resulting in overactivity of the parasympathetic nervous system. Medication for OPC poisoning consists of high-dose intravenously administered atropine to temporarily block these cholinergic effects plus an oxime (typically pralidoxime) to restore the activity of the acetylcholinesterase enzyme. Although there is no clear evidence of benefit from oxime therapy in OPC poisoning [5], atropine therapy is known to improve outcome [6, 7].
Although no standard regimen exists, there are two main approaches to determine the dosing schedule for atropine that are in widespread use. Both rely on the identification of clinical features of atropinization to determine when sufficient atropine has been administered. This is termed ‘individualized treatment’. The first and more conventional approach is bolus dose treatment with atropine initially every 15 min followed by lower doses at longer intervals until recovery. The second approach consists of more rapid atropinization with incremental boluses followed by an atropine infusion adjusted according to clinical response and tapered until recovery. From our experience, conventional bolus dose treatment is associated with delay in stabilization and ultimately a higher mortality compared to the second approach although this has not been formally investigated.
Thus, a study was done to compare the efficacy and safety of conventional bolus dose treatment with atropine for OPC poisoning with the incremental atropine dosing regimen followed by atropine infusion. The protocol used for each regimen was derived from reviews of the literature [8–12]. The objective of the study was to determine the optimal dosing regimen for atropine therapy in OPC poisoning. The primary outcome measure was mortality. Secondary outcome measures were time to atropinization, total dose of atropine required, incidence of atropine toxicity, incidence of intermediate syndrome (IMS) and duration of hospitalization. It was hoped the study would help to inform development of a future guideline for better management of OPC poisoning to improve outcome.
Materials and Methods
Study Design
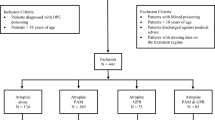
This was an open-label unblinded randomized clinical trial conducted with two equally sized parallel groups in the Department of Medicine, Chittagong Medical College Hospital (CMCH), Chittagong, Bangladesh, from June to September 2006. CMCH is a large tertiary referral hospital with limited facilities for artificial ventilation and renal dialysis. The study is summarized as a Cochrane Flow Diagram in Fig. 1. Inclusion criteria were patients with definite OPC poisoning by all of: (1) clear volunteered history of ingestion of OPC (irrespective of the nature of poisoning—deliberate self harm, homicidal or otherwise—and regardless of brand of OPC and whether or not a sample was provided for identification), (2) clear clinical features of toxicity (acute cholinergic crisis, i.e. any one of: pupils ≤2 mm diameter, fasciculations, heart rate <60 per minute, systolic blood pressure ≤80 mmHg, decreased consciousness (GCS <15), excessive sweating or poor air entry due to bronchorrhea and/or bronchospasm) and (3) pungent garlic odour in the mouth or from gastric aspirate. Exclusion criteria were (1) mild or no toxicity (determined by test dosing with atropine where necessary; for more details, please refer to “Treatment Procedure” in the following text), (2) any concomitant serious acute illness, (3) pregnancy or (4) patients not giving written informed consent to be in the study. Ethical permission for this study was provided by the Ethical Review Committee of Chittagong Medical College.
The patients were randomly divided into two groups according to cards drawn from a sealed box by a patient’s attendant at the time of enrollment. The sealed box contained equal numbers of cards with either “group A” or “group B” and a study code written on them. These two groups were then given atropine with different dosing regimens as described in detail in the following text and in Fig. 2.
Sample Size Calculation
With an assumed in-hospital mortality rate for OPC poisoning of around 30%, to achieve an absolute reduction to 10% with a significance level (alpha) of 5% and a power (beta) of 84%, a minimum of 69 patients were required in each arm of the trial (i.e. 138 patients in total), assuming no withdrawals or loss to follow-up.
Subject Enrollment
Study subjects were selected on the basis of history and physical examination. Specially trained hospital medical officers were recruited from the medical wards for monitoring the study patients and collecting clinical data. Written informed consent was sought from all enrolled subjects, and in those who were unable to do so through being too unwell or unconscious, it was sought from their attending relatives. Of these patients, those who recovered were asked if they wished to continue in the study. The patients were treated according to allocated groups A or B as shown in Fig. 2. Study doctors were unblinded as to which study group the patients were in as the nature of the treatments with frequent reviews and dose/interval adjustments made blinding difficult. Baseline data including age, sex, religion, occupation, education, marital status, circumstances of poisoning, type of poison used, nature of poisoning and treatment prior to hospitalization including prior gastric lavage (e.g. in primary health care centres or in the emergency department of CMCH) were recorded for all patients by conducting direct interviews with the patients and attending relatives. Data on timing and dose of atropinization and atropine toxicity were recorded in an atropinization observation chart as developed by Eddleston et al [13]. Once the target number (n = 69) had been recruited in both groups, a meeting of the investigators was held to review the adequacy of enrollment to date and it was agreed that recruitment be ceased.
Treatment Procedure
The algorithm for treatment used in the study is shown in Fig. 2. Overall, patient management was the responsibility of the ward doctors and study-specific treatments (A, B and oxime) were given by the study doctors working closely with the ward doctors. The initial treatment was establishment of a clear airway and adequate ventilation. High-flow oxygen was given if necessary and airways were maintained using a Guedel airway. The patients were kept in the left lateral position, with head down and neck extended to minimize aspiration. As most patients were clinically dehydrated, circulation was supported by giving 500–1,000 ml normal saline (10–20 ml/kg) over the first 10–20 min. Measures were taken to reduce absorption of OPC through skin and mucus membranes, i.e. washing with water and soap where available and removal of clothes contaminated with OPC. Vital signs and Glasgow Coma Score (GCS) were recorded at baseline and throughout the admission.
In persons with suspected mild poisoning, a ‘test dose’ of intravenous parenteral atropine sulphate (1 mg in adults) was given (Fig. 2). If signs of atropinization occurred rapidly, poisoning was considered to be mild and such individuals were not included in the study (Fig. 1). Both groups received parenteral atropine, as described in the following text, following predefined algorithms [10–12], during which they were monitored at least every 3 h.
Group A
Subjects in group A received increasing doses of intravenous atropine from 2 to 5 mg (1 ampoule = 0.6 mg of atropine in 1 ml) depending on severity, as assessed by the treating clinician. This was repeated every 10 to 15 min until signs of atropinization were clinically evident [11] (clear chest on auscultation with resolution of bronchorrhea, heart rate of >80 beats per minute, systolic blood pressure >80 mmHg, dry axillae and pupils >2 mm in diameter). The subsequent dosing with atropine injections was individualized either by decreasing the dose or increasing the duration in between doses as per the preference of the treating clinician, provided features of atropinization were still present. If one or all of these features were absent, the dose or frequency of atropine was increased as per the preference of the treating clinician. Atropinization was maintained for at least 24 h until clinical recovery, i.e. resolution of all features of cholinergic crisis listed earlier.
Group B
Individuals in the second group were treated according to a previously published algorithm [10]. They were given more rapidly incremental doses of bolus atropine, i.e. a first dose of 1.8–3.0 mg (3–5 ampoules) depending on severity as assessed by the treating clinician followed by another 5 min later at double this dose if needed. This was repeated every 5 min until all clinical signs of atropinization were clearly evident. After initial atropinization, patients were maintained on an atropine infusion, using 10% of the atropine required to load the patient given per hour (e.g. if atropine required for atropinization was 18 mg, 1.8 mg was infused each hour by mixing the amount of atropine required for 24 h with 1,000 ml normal saline and giving it at a rate of 40 micro drops per minute as a continuous infusion). After initial atropinization, patients were reviewed and assessed for the five features of atropinization (given earlier for group A) every 15 min. At each review, if the features of atropinization were not present, a further bolus of atropine was given (the amount decided by the treating clinician) and the infusion rate was also increased by 0.6 mg/h until the features appeared. Once this had occurred, patients were observed at least every hour (more frequently if between 3 and 5 mg/h of atropine was being given) for the first 6 h to check that the atropine infusion rate was sufficient and that there were no signs of atropine toxicity (the presence of all three of confusion, hyperthermia (axillary temperature >99°F) and absent bowel sounds). Thereafter, patients were seen at 2- to 3-h intervals if no complications were present. When atropine toxicity occurred, atropine infusion was stopped temporarily and the patient was checked after 30 min to see whether the features of toxicity had settled. If not, the treatment was withheld with further reviews every 30 min until atropine toxicity had resolved. Atropine was then restarted at 70–80% of the previous rate. The patient was observed frequently to ensure that the new infusion rate had reduced the signs of atropine toxicity without permitting the reappearance of cholinergic signs. This infusion was discontinued after a minimum of 24 h once all features of cholinergic crisis had resolved.
Treatment Common to Both Groups
Oximes were given to all patients with acute OPC poisoning who presented within 36 h of poisoning. The dose of pralidoxime was 30 mg/kg (1–2 g in adults, maximum dose of 2 g) given by slow intravenous injection at a maximum rate of 0.5 g/min. Treatment with pralidoxime was repeated as a bolus at the same dose and rate as the initial dose every 8 h for 48 h in all surviving patients as per standard practice in CMCH at the time. Evidence of pralidoxime toxicity including tachycardia, muscular rigidity, neuromuscular blockade, hypertension, laryngospasm and mild hepatitis was recorded and managed by reducing the subsequent dose of pralidoxime and/or atropine as appropriate. Other supportive treatments were given as per the desire of the treating clinician.
All patients were monitored for early signs of IMS due to OPC poisoning. Signs of IMS were weakness of neck flexion, difficulty in lifting the head off the pillow, use of accessory muscles of respiration, nasal flaring, tachypnea, sweating, cranial nerve palsies and proximal limb muscle weakness with retained distal muscle strength. IMS was managed by supportive measures including intubation and ventilation if required.
The patients were discharged after a minimum of 24 h of observation post-cessation of atropine if they had no residual features of OPC poisoning.
Statistical Analysis
A p value of ≤0.05 was considered as the minimum level of significance. The main analysis was carried out on an intention-to-treat basis, using the chi-square test and two-tailed t-test where appropriate. Comparison of the two groups at baseline was done using these same tests. To compare mortality, a Kaplan–Meier curve was used. Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) for Windows, version 15.0 (SPSS, Chicago, IL, USA).
Observations and Results
Features on Enrollment
There were 81 patients enrolled in group A and 75 in group B. Demographic history and pre-enrollment management for both groups are shown in Table 1. There were no significant differences between the two groups.
There was a wide range of times to hospitalization because some patients were treated initially in a peripheral Upazilla Health Complex and/or district hospital and then referred to CMCH, while others were admitted directly to CMCH.
Of the 143 study subjects who took a known substance, malathion was taken by 71 (49.7%), dichlorvos by 24 (16.8%), diazinon by 19 (13.3%), dimethoate by 18 (12.6%) and fentrothion by 11 (7.7%). There were no significant differences between the two groups in the type of organophosphate taken.
Table 2 shows the clinical features of OPC poisoning present on enrollment. There were no differences between the two groups.
Management
Table 3 shows the time to atropinization and total dose of atropine given to each group. Out of 81 patients in group A, five died before complete atropinization. The time to full atropinization for those in group A was much longer (6.35 times more) than for those in group B. The amount of atropine required was lower in group A. In group A, only one of 81 (1.3%) patients was atropinized within 60 min. In group B, two of 75 (2.7 %) were atropinized within 30 min and one of 75 (1.3%) in 30–60 min. The frequency of atropine toxicity was also compared between the groups. In group A 23/81 (28.4%) and in group B nine of 75 (12.0%) developed atropine toxicity (p = 0.011).
Outcome
Clinical outcomes are shown in Table 4. A total of 24 patients died. Mortality was lower in group B. Figure 3 shows a Kaplan–Meier plot of survival for each group.
Of 18 deaths in group A, 13 (72%) were due to respiratory failure and five (27.8%) were due to circulatory failure. Of six deaths in group B, three (50%) were due to respiratory failure, two (33.3%) due to circulatory failure and one (16.7%) due to diarrhoea complicated by shock. Mortality was higher in those with GCS ≤10 compared to those with GCS >10 on enrollment (13/27 (48.1%) vs 11/128 (8.6%), p < 0.0001).
Two patients in group A recovered with complications. One developed acute extensive anterior myocardial infarction and the other developed delayed neuropathy manifested by foot drop.
Atropine toxicity occurred in 32 patients, IMS in 14 and respiratory arrest or respiratory failure in 27. More patients in group A had atropine toxicity or IMS than in group B. Of those who developed IMS, only six received ICU support due to limited availability. The other eight patients with IMS were intubated in the general medical ward and ventilation was maintained manually by using an Ambu bag. These eight patients eventually died due to lack of ICU support. Of those patients who received ICU care, three of six (50%) survived. Those who died in ICU did so from failure of weaning and refractory circulatory failure.
Table 5 shows the duration of hospital stay and time to death. There were no differences in hospital stay or time to death between the groups. The longest hospital stay was 27 days. Most of the patients staying after 5 days required respiratory support. In group A, 15/18 (83.3%) died within 24 h of admission, and of these five (33.3%) died due to respiratory failure before atropinization was achieved. Of the remaining ten patients atropinization was achieved, but eight patients died due to respiratory failure and two patients due to respiratory failure plus circulatory failure. In group B, four of six (66.7%) patients who died had sudden respiratory arrest before intubation within the first 24 h of their admission. The other two (33.3%) patients died after 24 h, one of diarrhoea with shock and one died in the ICU after 17 days of admission due to respiratory failure plus circulatory failure.
Discussion
This study found that rapid atropinization followed by atropine infusion greatly reduced mortality when compared to standard treatment with boluses of atropine (22.2% vs 8%). It required a shorter time to atropinization and there was a lower incidence of atropine toxicity, although the total dose of atropine administered was a little greater. There was also less IMS with this regimen.
In group A, 22.5% of patients died, compared to 8% in group B. This is similar to a previous study in 45 cases of OPC poisoning which showed that continuous infusion significantly reduced mortality compared to intermittent boluses (23.5% to 8.8%; p < 0.05) [14]. The overall mortality rate from OPC poisoning in this study was 15.4%. This is marginally lower than in a previous study in the same institution (16.7%) [1] and in a variety of other settings (20–25%) [15–19]. The present study found that those with lower GCS on enrolment had much higher mortality as found previously [20].
Intravenous high-dose atropine is an established life-saving component of the initial management of OPC poisoning. However, there remains much discussion as to the optimal dosing regimen and numerous variations are in use around the world. In spite of an increasing frequency of suicidal attempts with OPCs, few studies have been undertaken to rationalize therapy for OPC poisoning and there remains great scope for reducing the high mortality through optimizing therapy.
The relatively few formal trials in OPC poisoning that have been done make it clear that early antagonism of OPC toxicity is associated with better outcome [14, 21–23]. Full and early atropinization is ideal as delayed atropinization can result in avoidable death from central respiratory depression, bronchospasm, bronchorrhoea, severe bradycardia and/or hypotension [24]. In group A, five patients died before atropinization was achieved. It is likely that the accelerated regimen employed in arm B of this study was responsible for the difference in mortality and complications. Conventional bolus dose atropine treatment of OPC poisoning, the most frequently used regimen, was postulated and found in this study to be associated with delay in stabilization of poisoned patients, more atropine toxicity and ultimately more fatality.
The time to atropinization of group A was much (6.35 times) longer than that of group B (p < 0.001) although the average requirement of atropine was found to be a little greater in group B. With treatment B, the rapidly incremental dosing regimen, atropinization could be achieved within a very short time. Although there are few studies on the subject, there is some evidence that patients in the developing world often die soon after admission [25]. The rapid and effective stabilization and treatment of pesticide poisoned patients on admission should reduce the number of early deaths, improve the prognosis for surviving patients over the first few days and reduce the number and severity of long-term sequelae.
The present study compared the conventional bolus dose atropine treatment of OPC poisoning with incremental dose atropine treatment followed by atropine infusion. In clinical practice, dosage regimens are usually designed according to severity of poisoning and to the signs of atropinization [1]. In the present study, persons with mild poisoning were excluded; otherwise, no discrimination was made with respect to severity, with all moderate–severe patients being included and strictly randomized. Due to there being no accepted grading scale for the severity of OPC poisoning, there was no attempt to formally grade severity of poisoning in enrolled patients.
The majority of enrolled patients (78.2%) were less than 30 years of age and the two groups were broadly similar in terms of demographics, prehospital treatment received, time to reach hospital and presenting features. A majority (91.7%) of the subjects took substances which were known and most (91.7%) poisonings were self-inflicted. In Bangladesh, OPCs are the commonest chemical agents used for attempted suicide [26, 27].
The time interval between exposure to OPC and onset of symptoms of poisoning varies with the route and degree of exposure [2]. Following massive ingestions, the symptoms appear within several minutes [25]. With smaller amounts, in most instances, symptoms appear within 30 min of exposure and almost always in less than 12 h [2]. In our series, the earliest presentation of an individual with features of toxicity was within 30 min of ingestion of OPC, although 25% took more than 4 h to reach the hospital. Delay in discovery and transport to the hospital as well as differences in treatment-seeking behaviour can cause differences in presentation and increased mortality. Local effects on eyes and respiratory tract may appear within minutes. After ingestion of OPC, the initial symptoms may be gastrointestinal or may be referable to any of the other organs affected [2]. In the present series, pupillary and glandular manifestations were almost invariably present.
IMS occurs due to dysfunction of the post-synaptic neuromuscular junction [28]. It is a neurological complication affecting many patients with OPC poisoning during conventional treatment or on recovery from acute cholinergic crisis. In the present study, IMS developed in 14 patients among whom the offending OPC agent was identified in eight of these, four ingested malathion, two ingested dichlorvos and two ingested dimethoate. Regimen B was found to be associated with a lower risk of developing IMS (p < 0.05). The pathogenesis of IMS is not understood but it is thought to be due to persistent inhibition of acetylcholinesterase. Theoretically, therefore, its incidence should be unaffected by atropine but may be reduced by effective oxime therapy, although evidence for this is lacking. The difference in incidence of IMS between the two groups in the present study suggests that an alternative mechanism may be contributing. No clinical trial which was adequately powered to explore this has yet been undertaken. A difference in IMS between the two groups may alternatively reflect an imbalance between the two groups, although there were no other indications of this from the analysis.
Respiratory support was needed for 26 patients but only available for six due to resource limitations. Of these six, half survived. In other studies, the survival rate in OPC poisoning requiring mechanical ventilation varied between 13% and 50% in a variety of settings [29–31]. Respiratory support was required in significantly fewer patients treated with regimen B. Again, this was probably due to earlier reversal of cholinergic features by rapid atropinization. The cause of respiratory failure in OPC poisoning is multi-factorial, including bronchorrhea, bronchospasm, weakness of respiratory muscles and centrally mediated respiratory depression. In a better resourced setting, it is possible that the availability of a larger, well-equipped ICU facility may have reduced the overall mortality in this trial. It is not clear therefore whether the difference in mortality between these two regimens would also apply to such a well-resourced setting.
To examine safety, the occurrence of atropine toxicity also compared between the treatment groups. Patients treated with conventional bolus dose were found to be more at risk of developing atropine toxicity (28.4% vs 12.0%, p < 0.05). Previous studies have found similar rates with bolus regimens (26%) [32]. This was probably due to repeated bolus dosing with atropine and also due to failure to reduce the dose of atropine quickly. A recent trial comparing ad hoc bolus doses with titrated doses found ad hoc dosing to result in more and higher doses of atropine, more atropine toxicity and longer hospital stays [33]. In conventional bolus dosing regimens, after initial atropinization, it is not clear as to how frequently to follow up the patient and how quickly and how much to reduce the dose of atropine. The lower incidence of atropine toxicity in group B patients was probably partly due to the continuous infusion of atropine producing little fluctuation of blood levels.
The study had several limitations. Study doctors were not blinded as to which treatment group patients were in. This was for practical purposes as these doctors had to assist with managing the different treatment regimens, including performing clinical assessments and adjustment of atropine dosing as per the protocol. This may have been a source of bias in the assessment of the subjective outcome measures, although clear definitions for atropine toxicity, atropinization and IMS were adhered to as strictly as possible. The other outcome measures, mortality and duration of hospitalization, were less likely to be affected by bias from non-blinding. Testing was not done to assess for possible confounders in the comparison of outcome measures. Overall, patient management was the responsibility of the ward doctors who also decided about patient discharge.
It was not possible to perform assays for cholinesterase activity or direct measurement of OPC concentrations in this study as they were unavailable to the study team. A diagnosis of OPC poisoning was thus based on history and examination alone and only those with moderate–severe features were included. The criteria used were strict and the likelihood of a patient having all these features but an alternative diagnosis was thought to be low. The utility of cholinesterase activity without a baseline pre-poisoning level is also limited.
In summary, atropine for OPC poisoning given by individualized incremental bolus doses followed by continuous infusion has several advantages over conventional incremental bolus doses alone. Early stabilization reduces short term mortality. Conventional bolus dosing regimens require more frequent follow-up to monitor for atropinization and atropine toxicity and this is impractical in very busy resource-limited settings. Adoption of a regimen that results in rapid atropinization will likely save significant numbers of lives across the developing world where junior doctors manage patients without advice from clinical toxicologists. Recommended regimens must be simple and easily used by such doctors.
With the incremental dosing regimen, initial stabilization can be achieved within a very short time, followed by continuous atropine infusion, allowing less frequent follow-up, sustained blood levels of atropine with little chance of fluctuation and ultimately less toxicity and lower mortality.
References
Faiz MA, Rahman MR, Ahmed T (1994) Management of acute poisoning with organophosphorus insecticide. J Bang Coll Phys Surg 12:59–62
Eddleston M (2000) Patterns and problems of deliberate self-poisoning in the developing world. Q J Med 93:715–731
Eddleston M, Phillips MR (2004) Self poisoning with pesticides. BMJ 328:42–44
Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M (2004) Where is the evidence for the management of pesticide poisoning—is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol 42:113–116
Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev 2011; CD005085.
Blain PG. Organophosphorus poisoning (acute). Clin Evid (Online). 2011; 2011: 2102.
Eddleston M, Buckley NA, Eyer P, Dawson AH (2008) Management of acute organophosphorus pesticide poisoning. Lancet 371:597–607
Eddleston M, Singh S, Buckley N (2003) Acute organophosphorus poisoning. Clinical Evidence 10:1652–1663
Eddleston M, Szinicz L, Eyer P, Buckley N (2002) Oximes in acute organophosphorus pesticide poisoning: a systematic review of clinical trials. Q J Med 95:275–283
Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A et al (2004) Early management after self poisoning with an organophosphorus or carbamate pesticide—a treatment protocol for junior doctors. Crit Care 8:391–396
Lotti M (1991) Treatment of acute organophosphorus poisoning. Med J Aug 154:51–55
Minton NA, Murray VS (1988) A review of organophosphorus poisoning. Med Toxicol 3:350–357
Eddleston M, Buckley NA, Dawson AH et al (2004) Speed of initial atropinisation in significant organophosphorus pesticide poisoning—a systematic comparison of recommended regimens. J Toxicol Clin Toxicol 42:865–875
Sunder Ram J, Kumar SS, Jayarajan A, Kuppuswamy G (1991) Continuous infusion of high doses of atropine in the management of organophosphorus compound poisoning. J Asso Phys India 39:190–193
Karalliedde L, Senanayake N (1988) Pattern of acute poisoning in a medical unit in central Sri Lanka. Forensic Sci Int 36:101–104
Jeyaratnam J, de Alwis Seneviratne RS, Copplestone JF (1982) Survey of pesticide poisoning in Sri Lanka. Bull WHO 60:615–619
Wyckoff DW, Davies JE, Barquet A, Davis JH (1968) Diagnostic and therapeutic problems of parathion poisonings. Ann Int Med 68:875–882
Yamashita M, Tanaka J, Ando Y (1997) Human Mortality in organophosphate poisonings. Vet Hum Toxicol 39:84–85
Abdollahi M, Jalali N, Sabzevari O, Hoseini R, Ghanea T (1997) A retrospective study of poisoning in Tehran. J Clin Toxicol 35:387–393
Davies JO, Eddleston M, Buckley NA (2008) Predicting outcome in acute organophosphorus poisoning with a poison severity score or the Glasgow coma scale. QJM 101:371–9
Bird SB, Gaspari RJ, Dickson EW (2003) Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med 10:295–298
Dickson EW, Bird S, Gaspari RJ, Boyer EW, Ferris DF (2003) Diazepam inhibits organophosphate-induced central respiratory depression. Acad Emerg Med 10:1303–1306
Basu A, Das AK, Chandrasekar S (1988) Organophosphorus poisoning—a clinical profile. J Asso Phys India 36:24
Ballantyne B, Marrs TC (1992) Overview of the biological and clinical aspects of organophosphates and carbamates. In: Clinical and experimental toxicology of organophosphates and carbamates. Butterworth Heinemann, Oxford, pp 3–14
de Alwis LBL, Salgado MSL (1988) Agrochemical poisoning in Sri Lanka. Forensic Sci Int 36:81–89
Alam MS (1995) Agrochemicals and its medicolegal hazards a retrospective study at Sher-E-Bangla Medical College, Barisal from 1990–1992. J Teachers Asso SBMC & H, Bang 6:367–369
Faiz MA (2004) An urgent need for improvement of management of poisoning (editorial). J Dhaka Med Coll 13:1–3
Jayawardane P, Dawson AH, Weerasinghe V, Karalliedde L, Buckley NA, Senanayake N (2008) The spectrum of intermediate syndrome following acute organophosphate poisoning: a prospective cohort study from Sri Lanka. PLoS Med 5:e147
Bardin PG, van Eeden SF, Joubert JR (1987) Intensive care management of acute organophosphate poisoning: a 7-year experience in the Western Cape. S Afr Med J 72:593–597
Rajapakse VP, Wijesekera S (1989) Outcome of mechanical ventilation in Sri Lanka. Ann R Coll Surg Engl 71:344–346
Yen DH, Yien HW, Wang LM, Lee CH, Chan SH (2000) Spectral analysis of systemic arterial pressure and heart rate signals of patients with acute respiratory failure induced by severe organophosphate poisoning. Crit Care Med 28:2805–2811
World Health Organization. International programme on chemical safety evaluation. Antidotes for poisoning by organophosphorus pesticides. Monograph on Atropine. 2002. (Accessed at http://www.inchem.org/documents/antidote/antidote/atropine.htm on 17th October 2011)
Perera PM, Shahmy S, Gawarammana I, Dawson AH (2008) Comparison of two commonly practiced atropinization regimens in acute organophosphorus and carbamate poisoning, doubling doses vs. ad hoc: a prospective observational study. Hum Exp Toxicol 27:513–8
Acknowledgements
The authors would like to thank the staff and patients at Chittagong Medical College Hospital, without whom this study would not have been possible. This study was funded by Chittagong Medical College.
Conflicts of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abedin, M.J., Sayeed, A.A., Basher, A. et al. Open-Label Randomized Clinical Trial of Atropine Bolus Injection Versus Incremental Boluses Plus Infusion for Organophosphate Poisoning in Bangladesh. J. Med. Toxicol. 8, 108–117 (2012). https://doi.org/10.1007/s13181-012-0214-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-012-0214-6