Abstract
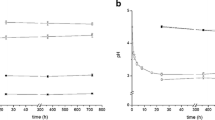
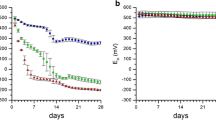
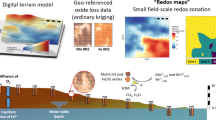
Characterization of the soil redox status is important for pedogenesis but simple field methods for monitoring are limited. Recently, we introduced manganese (MnIII,IV) oxide-coated redox bars as an indicator for reducing conditions in soils. In this study, we compared these redox bars with well-established iron (FeIII) oxide-coated bars. For a 5-month monitoring period, we quantified the monthly oxide removal along three wetland plots with different variations in water table. Preferential dissolution of the Mn oxide coating exceeded the Fe oxide removal by two to five times that is coherent with the thermodynamic stability of the minerals. Enhanced removal of Mn oxide coatings in the capillary fringe compared to minor depletion of Fe oxides enables to differentiate weakly (300 to 100 mV, range of MnIII,IV reduction) and moderately (100 to −100 mV, range of FeIII reduction) reducing conditions. Processes that occur under weakly reducing soil conditions, e.g. denitrification and trace metal mobilization associated with the reductive dissolution of Mn oxides, can be identified when Mn oxide removal along redox bars occurs but the Fe oxide coating remains stable. Simultaneous use of Mn and Fe redox bars results in a better temporal and spatial characterization of the soil redox status.




Similar content being viewed by others
Abbreviations
- EH :
-
redox potential
- WT:
-
water table
- CWB:
-
climatic water balance
References
Bezbaruah AN, Zhang TC (2004) pH, redox, and oxygen microprofiles in rhizosphere of bulrush (Scirpus validus) in a constructed wetland treating municipal wastewater. Biotechnol Bioeng 88:60–70. doi:10.1002/bit.20208
Bormann H, Diekkrüger B, Richter O (1996) Effects of data availability on estimation of evapotranspiration. Phys Chem Earth 21:171–175. doi:10.1016/S0079-1946(97)85580-2
Castenson KL, Rabenhorst MC (2006) Indicator of reduction in soil (IRIS): evaluation of a new approach for assessing reduced conditions in soil. Soil Sci Soc Am J 70:1222–1226. doi:10.2136/sssaj2005.0130
Chapelle FH, McMahon PB, Dubrovsky NM, Fujii RF, Oaksford ET, Vroblesky DA (1995) Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resour Res 31:359–371. doi:10.1029/94WR02525
Childs CW (1981) Field-tests for ferrous iron and ferric-organic complexes (on exchange sites or in water soluble forms) in soils. Aust J Soil Res 19:175–180. doi:10.1071/SR9810175
Davydov A, Chuang KT, Sanger AR (1998) Mechanism of H2S oxidation by ferric oxide and hydroxide surfaces. The Journal of Physical Chemistry 102:4745–4752. doi:10.1021/jp980361p
Dorau K, Mansfeldt T (2015) Manganese-oxide–coated redox bars as an indicator of reducing conditions in soils. J Environ Qual 44:696–703
Della Puppa K, Momarek M, Bordas F, Bollinger JC, Joussein E (2013) Adsorption of copper, cadmium, lead and zinc onto a synthetic manganese oxide. J Colloid Interface Sci 399:99–106. doi:10.1016/j.jcis.2013.02.029
Fakih M, Davranche M, Dia A, Nowack B, Petitjean P, Chatellier X, Gruau G (2008) A new tool for in situ monitoring of Fe-mobilization in soils. Appl Geochem 23:3372–3383. doi:10.1016/j.apgeochem.2008.07.016
Fiedler S, Vepraskas MJ, Richardson JL (2007) Soil redox potential: importance, field measurements, and observations. In: Sparks DL (ed) Advances in Agronomy. Elsevier Academic Press Inc, San Diego, pp. 1–54
IUSS Working Group WRB, 2006. World reference base for soil resources. World Soil Resources Reports No. 103, FAO, Rome
Jenkinson BJ, Franzmeier DP (2006) Development and evaluation of iron-coated tubes that indicate reduction in soils. Soil Sci Soc Am J 70:183–191. doi:10.2136/sssaj2004.0323
Kirk G (2004) The biogeochemistry of submerged soils. John Wiley & Sons, Hoboken
Mansfeldt T, Overesch M (2013) Arsenic mobility and speciation in a Gleysol with petrogleyic properties: a field and laboratory approach. J Environ Qual 42:1130–1141. doi:10.2134/jeq2012.0225
Ottow JCG (2011) Microbiology of soils. Springer, Heidelberg
Owens PR, Wilding LP, Miller WM, Griffin RW (2008) Using iron metal rods to infer oxygen status in seasonally saturated soils. Catena 73:197–203. doi:10.1016/j.catena.2007.07.009
Patrick WH, Gambrell RZ, Faulkner SP (1996) Redox measurements of soils. In: Sparks DL et al. (eds) Methods of soil analysis: chemical methods part 3. Soil Science Society of America and American Society of Agronomy, Madison, pp. 1255–1273
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96. doi:10.1016/S0065-2113(08)60633-1
Rabenhorst MC, Castenson KL (2005) Temperature effects on iron reduction in a hydric soil. Soil Sci 170:734–742. doi:10.1097/01.ss.0000185908.26083.53
Rabenhorst MC (2010) Visual assessment of IRIS tubes in field testing for soil reduction. Wetlands 30:847–852. doi:10.1007/s13157-010-0098-7
Rabenhorst MC, Megonigal JP, Keller J (2010) Synthetic iron oxides for documenting sulfide in marsh pore water. Soil Sci Soc Am J 74:1383–1388. doi:10.2136/sssaj2009.0435
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, Boca Raton
Ringrose-Voase AJ, Humphreys GS (1993) Soil micromorphology: studies in management and genesis. Developments in Soil Science, 22. Elsevier, Amsterdam
Römheld V (1991) The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: an ecological approach. Plant Soil 130:127–134. doi:10.1007/BF00011867
Schlesinger WH, Emily SB (2013) Biogeochemistry: an analysis of global change, 3rd edn. Academic Press, Amsterdam
Scott MJ, Morgan JJ (1990) Energetics and conservative properties of redox systems. In: American Chemical Society (ed) Chemical modeling of aqueous systems II. ACS Symposium Series, pp 368–378
Smith KA (1980) A model of the extent of anaerobic zones in aggregated soils, and its potential application to estimates of denitrification. J Soil Sci 31:263–277
Stiles CA, Dunkinson ET, Ping CL, Kidd J (2010) Initial field installation of manganese indicators of reduction in soils, Brooks Range, Alaska. Soil Survey Horizons 51:102–107
Treeby M, Marschner H, Römheld V (1989) Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 114:217–226. doi:10.1007/BF02220801
Wheeler BD, Al-Farraj MM, Cook RED (1985) Iron toxicity to plants in base-rich wetlands: comparative effect on the distribution and growth of Epilobium Hirsutum L. and Juncus Subnodulosus Schrank. New Phytol 100:653–669. doi:10.1111/j.1469-8137.1985.tb02810.x
Yu K, Patrick WH (2004) Redox window with minimum global warming potential contribution from rice soils. Soil Sci Soc Am J 68:2086–2091. doi:10.2136/sssaj2004.2086
Acknowledgments
This study was financially supported by Verein der Freunde und Förderer der Universität zu Köln. Additionally, we are grateful to the Duke of Croy and Thomas Seine, who enabled the field measurements along the study site.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorau, K., Eickmeier, M. & Mansfeldt, T. Comparison of Manganese and Iron Oxide-Coated Redox Bars for Characterization of the Redox Status in Wetland Soils. Wetlands 36, 133–141 (2016). https://doi.org/10.1007/s13157-015-0724-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0724-5