Abstract
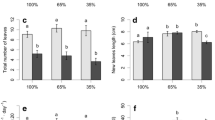
A field survey was performed to examine nonstructural carbohydrate (NSC) dynamics in seagrass Thalassia hemprichii at the Xincun Bay in southern China. An indoor experiment to investigate the response of NSC in T. hemprichii to shade was conducted. Belowground tissue of T. hemprichii was the dominant site of NSC reserves, and soluble sugar was the primary storage compound. The starch content of belowground tissue was lower in high intertidal areas than in low intertidal areas, indicating that the longer air exposure in high intertidal areas resulted in less NSC synthesis and less accumulation of NSC in T. hemprichii. The lowest level of soluble sugar and its proportion to NSC in belowground tissue were observed near the cage culture area, where the nutrient concentration in water and sediment was the highest; while the highest level of that was observed near the coastal shrimp farm, Where salinity was the lowest. Soluble sugar in belowground tissue showed the following trend: summer>spring>winter>autumn. This corresponded to seasonal changes in the intensity of light. Leaf sugar accumulated during the autumn-winter period, providing a carbon and energy source for flower bud formation and seed germination. Short-term shading decreased NSC accumulation. Collectively, these results suggest that nutrient enrichment, freshwater discharge and exposure to air affect NSC dynamics in T. hemprichii. Light intensity, flower bud formation, and seed germination were all found to induce seasonal variations in NSC in T. hemprichii.
Similar content being viewed by others
References
Alcoverro T, Manzanera M, Romero J. 2001. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Marine Ecology Progress Series, 211: 105–116
Alcoverro T, Zimmerman R, Kohrs D, et al. 1999. Resource allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Marine Ecology Progress Series, 187: 121–131
Biber P D, Kenworthy W J, Paerl H W. 2009. Experimental analysis of the response and recovery of Zostera marina (L.) and Halodule wrightii (Ascher.) to repeated light-limitation stress. Journal of Experimental Marine Biology and Ecology, 369(2): 110–117
Björk M, Short F, McLeod E, et al. 2008. Managing Seagrasses for Resilience to Climate Change. Gland: International Union for Conservation of Nature and Natural Resources, 56
Brun F G, Olive I, Malta E J, et al. 2008. Increased vulnerability of Zostera noltii to stress caused by low light and elevated ammonium levels under phosphate deficiency. Marine Ecology Progress Series, 365: 67–75
Burke M K, Dennison W C, Moore K A. 1996. Non-structural carbohydrate reserves of eelgrass Zostera marina. Marine Ecology Progress Series, 137: 195–201
Burkholder J A M, Mason K M, Glasgow J H B. 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: Evidence from seasonal mesocosm experiments. Marine Ecology Progress Series, 81: 163–178
Burkholder J A M, Tomasko D A, Touchette B W. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology, 350(1–2): 46–72
Cabaco S, Santos R. 2007. Effects of burial and erosion on the seagrass Zostera noltii. Journal of Experimental Marine Biology and Ecology, 340(2): 204–212
Chollett I, Bone D, Pérez D. 2007. Effects of heavy rainfall on Thalassia testudinumbeds. Aquatic Botany, 87(3): 189–195
Collier C J, Lavery P, Ralph P, et al. 2009. Shade-induced response and recovery of the seagrass Posidonia sinuosa. Journal of Experimental Marine Biology and Ecology, 370(1–2): 89–103
Collier C J, Waycott M, Ospina A G. 2011. Responses of four Indo-West Pacific seagrass species to shading. Marine Pollution Bulletin, 65: 342–354
De Rosa S, Zavodnik N, De Stefano S, et al. 1990. Seasonal Changes of biomass and soluble carbohydrates in the seagrass Zostera noltii Hornem. Botanica Marina, 33(5): 411–414
Dennison W C. 2009. Global trajectories of seagrasses, the biological sentinels of coastal ecosystems. In: Duarte CM, ed. Global Loss of Coastal Habitats: Rates, Causes and Consequences. Madrid: Fundacion BBVA, 91–108
Duarte C M, Gattuso J. 2010. Seagrass meadows. In: Cleveland C J, ed. Encyclopedia of Earth. Washington D C: Environmental Information Coalition, National Council for Science and the Environment
Eklöf J, McMahon K, Lavery P. 2009. Effects of multiple disturbances in seagrass meadows: shading decreases resilience to grazing. Marine and Freshwater Research, 60(12): 1317–1327
Fokeera-Wahedally S, Bhikajee M. 2005. The effects of in situ shading on the growth of a seagrass, Syringodium isoetifolium. Estuarine, Coastal and Shelf Science, 64(2–3): 149–155
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Aministration of the People’s Republic of China. 2008. GB17378.4-2007. The specification for marine monitoring, part 4: seawater analysis (in Chinese). Beijing: the Standards Press of China
Huang Xiaoping, Huang Liangmin, Li Yinghong, et al. 2006. Main seagrass beds and threats to their habitats in the coastal sea of South China. Chinese Science Bulletin, 51: 136–142
Invers O, Kraemer G, Pérez M, et al. 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology, 303(1): 97–114
Jupp B, Durako M, Kenworthy W, et al. 1996. Distribution, abundance, and species composition of seagrasses at several sites in Oman. Aquatic Botany, 53(3–4): 199–213
Koops A J, Groeneveld HW. 1990. Mobilization of endosperm reserves and uptake of sucrose and valine by the cotyledons of Euphorbia lathyris L. Journal of Experimental Botany, 41(10): 1279–1285
Lazar A C, Dawes C J. 1991. A seasonal study of the seagrass Ruppia maritima L. in Tampa Bay, Florida. Organic constituents and tolerances to salinity and temperature. Botanica Marina, 34: 265–269
Lee K S, Dunton K. 1996. Production and carbon reserve dynamics of the seagrass Thalassia testudinum in Corpus Christi Bay, Texas, USA. Marine Ecology Progress Series, 143: 201–210
Lee K S, Dunton K. 1997. Effect of in situ light reduction on themaintenance, growth and partitioning of carbon resources in Thalassia testudinum banks ex König. Journal of Experimental Marine Biology and Ecology, 210(1): 53–73
Lee K S, Park S R, KimY K. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. Journal of Experimental Marine Biology and Ecology, 350(1–2): 144–175
Leoni V, Vela A, Pasqualini V, et al. 2008. Effects of experimental reduction of light and nutrient enrichments (N and P) on seagrasses: a review. Aquatic Conservation: Marine and Freshwater Ecosystems, 18(2): 202–220
Longstaff B, Dennison W. 1999. Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquatic Botany, 65: 105–121
Mackey P, Collier C, Lavery P. 2007. Effects of experimental reduction of light availability on the seagrass Amphibolis griffithii. Marine Ecology Progress Series, 342: 117–126
Manzanera M, Pérez M, Romero J. 1998. Seagrass mortality due to oversedimentation: an experimental approach. Journal of Coastal Conservation, 4(1): 67–70
Marbà N, Duarte CM. 2010. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Global Change Biology, 16(8): 2366–2375
Murphy L R, Kinsey S T, Durako M J. 2003. Physiological effects of short-term salinity changes on Ruppia maritima. Aquatic Botany, 75(4): 293–309
Neckles H A, Short F T, Barker S, et al. 2005. Disturbance of eelgrass Zostera marina by commercial mussel Mytilus edulis harvesting in Maine: dragging impacts and habitat recovery. Marine Ecology Progress Series, 285: 57–73
Nejrup L B, Pedersen MF. 2008. Effects of salinity and water temperature on the ecological performance of Zostera marina. Aquatic Botany, 88(3): 239–246
Olivé I, Brun F G, Vergara J J, et al. 2007. Effects of light and biomass partitioning on growth, photosynthesis and carbohydrate content of the seagrass Zostera noltii Hornem. Journal of Experimental Marine Biology and Ecology, 345: 90–100
Palacios S, Zimmerman R. 2007. Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series, 344: 1–13
Peralta G, Pérez-Lloréns J, Hernández I, et al. 2002. Effects of light availability on growth, architecture and nutrient content of the seagrass Zostera noltii Hornem. Journal of Experimental Marine Biology and Ecology, 269(1): 9–26
Pirc H. 1985. Growth dynamics in Posidonia oceanica (L.) Delile. Marine Ecology, 6(2): 141–165
Pirc H. 1989. Seasonal changes in soluble carbohydrates, starch, and energy content in Mediterranean seagrasses. Marine Ecology, 10(2): 97–105
Pollard P, Greenway M. 1993. Photosynthetic characteristics of sea-grasses (Cymodocea serrulata, Thalassia hemprichii and Zoster-a capricorni) in a low-light environment, with a comparison of leaf-marking and lacunal-gas measurements of productivity. Australian Journal of Marine and Freshwater Research, 44(1): 127–139
Quarmby C, Allen S E. 1989. Organic Constituents. Oxford: Blackwell Scientific Press
Ralph P, Durako M, Enriquez S, et al. 2007. Impact of light limitation on seagrasses. Journal of Experimental Marine Biology and Ecology, 350(1–2): 176–193
Romero J, Martinez-Crego B, Alcoverro T, et al. 2007. Amultivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Marine Pollution Bulletin, 55: 196–204
Ruíz J, Marín-Guirao L, Sandoval-Gil J. 2009. Responses of the Mediterranean seagrass Posidonia oceanica to in situ simulated salinity increase. Botanica Marina, 52(5): 459–470
Ruíz J, Romero J. 2001. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Marine Ecology Progress Series, 215: 107–120
Serrano O, Mateo M, Renom P. 2011. Seasonal response of Posidonia oceanica to light disturbances. Marine Ecology Progress Series, 423: 29–38
Shafer D J, Sherman T D, Wyllie-Echeverria S. 2007. Do desiccation tolerances control the vertical distribution of intertidal seagrasses?. Aquatic Botany, 87(2): 161–166
Short F, Carruthers T, Dennison W, et al. 2007. Global seagrass distribution and diversity: A bioregionalmodel. Journal of Experimental Marine Biology and Ecology, 350(1–2): 3–20
Sugiura H, Hiroe Y, Suzuki T, et al. 2009. The carbohydrate catabolism of Zostera marina influenced by lower salinity during the pregermination stage. Fisheries Science, 75(5): 1205–1217
Touchette B W. 2007. Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology, 350(1–2): 194–215
Touchette B W, Burkholder J A M. 2002. Seasonal variations in carbon and nitrogen constituents in eelgrass (Zostera marina L.) as influenced by increased temperature and water-column nitrate. Botanica Marina, 45: 23–34
Uy W. 2001. Functioning of Philippine Seagrass Species Under Deteriorating Light Conditions. Boca Raton: the Chemical Rubber Company Press
Waycott M, Collier C, McMahon K, et al. 2007. Vulnerability of sea-grasses in the Great Barrier Reef to climate change. In: Johnson J E, Marshall P A, eds. Climate Change and the Great Barrier Reef. Townsville: Great Barrier Reef Marine Park Authority and Australian Greenhouse Office, 193–235
Xu Zhanzhou, Huang Liangmin, Huang Xiaoping, et al. 2008. A primary study on sexual reproduction of seagrass Thalassia hemprichii at Xincun Bay. Journal of Tropical Oceanography (in Chinese), 27(2): 60–63
Yemm E, Willis A. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal, 57: 508–514
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The National Natural Science Foundation of China under contract Nos 41076069 and 40776086; the Project of Environmental Quality Evaluation of Seagrass Bed in South China Sea under contract No. DOMEP (MEA)-01-03; the Public Science and Technology Research Funds Projects of Ocean under contract No. 201305030; the Knowledge Innovation Program of the Chinese Academy of Sciences under contract No. SQ201219.
Rights and permissions
About this article
Cite this article
Jiang, Z., Huang, X. & Zhang, J. Dynamics of nonstructural carbohydrates in seagrass Thalassia hemprichii and its response to shading. Acta Oceanol. Sin. 32, 61–67 (2013). https://doi.org/10.1007/s13131-013-0342-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-013-0342-0