Abstract
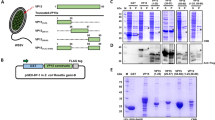
Recombinant bacteria secreting the transmembrane-truncated region of white spot syndrome virus (WSSV) envelope protein VP28 named DhpVB were constructed, using live Escherichia coli as a deliverer and a constitutive secretory expression plasmid as the expression vector. With the ability to deliver recombinant protein products outside, DhpVB couldmake VP28 directly interact with the shrimp when injected into shrimp. In order to test the protection potential, live DhpVB were injected into the marine shrimp Exopalamon carincauda Holthuis for three times and WSSV challenge was performed twice simultaneously at the last two injections of the bacteria. The results showed that DhpVB displayed statistically significant advantage over bacteria without VP28 after the second challenge with an average RI (Resistance index) of 0.67±0.08, compared with 0.41±0.09 of bacteria without VP28 (P <0.05). The data suggested that a quasi-immune response may exist in E. carincauda and live bacteria carrying VP28 could enhance the shrimp resistance against WSSV.
Similar content being viewed by others
References
Caipang C M A, Verjan N, Ooi E L, et al. 2008. Enhanced survival of shrimp, Penaeus (Marsupenaeus) japonicus from white spot syndrome disease after oral administration of recombinant VP28 expressed in Brevibacillus brevis. Fish & Shellfish Immunology, 25(3): 315–320
Chou H Y, Huang C Y, Wang C H, et al. 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Diseases of Aquatic Organisms, 23(3): 165–173
Du Huahua, Xu Zirong, Wu Xiaofeng, et al. 2006. Increased resistance to white spot syndrome virus in Procambarus clarkii by injection of envelope protein VP28 expressed using recombinant baculovirus. Aquaculture, 260(1–4): 39–43
Fu Linglin, Li Weifen, Du Hua Hua, et al. 2008. Oral vaccination with envelope protein VP28 against white spot syndrome virus in Procambarus clarkii using Bacillus subtilis as delivery vehicles. Letters in Applied Microbiology, 46(5): 581–586
Iwanaga S, Kawabata S I, Muta T. 1998. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: Their structures and functions. Journal of biochemistry, 123(1): 1–15
Jha R K, Xu Zirong R, Bai Shijuan, et al. 2007. Protection of Procambarus clarkii against white spot syndrome virus using recombinant oral vaccine expressed in Pichia pastoris. Fish & Shellfish Immunology, 22(4): 295–307
Johnson K N, van Hulten M C W, Barnes A C. 2008. “Vaccination” of shrimp against viral pathogens: Phenomenology and underlyingmechanisms. Vaccine, 26(38): 4885–4892
Kim Y S, Ryu J H, Han S J, et al. 2000. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. Journal of Biological Chemistry, 275(42): 32721
Kumar S, Ishaq Ahamed V P, Sarathi M, et al. 2008. Immunological responses of Penaeus monodon to DNA vaccine and its efficacy to protect shrimp against white spot syndrome virus (WSSV). Fish & Shellfish Immunology, 24(4): 467–478
Kumar S, Venkatesan C, Sarathi M, et al. 2009. Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish & Shellfish Immunology, 26(3): 429–437
Kurata S, Ariki S, Kawabata S I. 2006. Recognition of pathogens and activation of immune responses in Drosophila and horseshoe crab innate immunity. Immunobiology, 211(4): 237–249
Kuzyk MA, Burian J, Machander D, et al. 2001. An efficacious recombinant subunit vaccine against the salmonid rickettsial pathogen Piscirickettsia salmonis. Vaccine, 19(17–19): 2337–2344
Li Lijuan, Yuan Junfa, Cai Chengguang, et al. 2006. Multiple envelope proteins are involved in white spot syndrome virus (WSSV) infection in crayfish. Archives of Virology, 151(7): 1309–1317
Lin J H Y, Chen T Y, Chen M S, et al. 2006. Vaccination with three inactivated pathogens of cobia (Rachycentron canadum) stimulates protective immunity. Aquaculture, 255(1–4): 125–132
Lopez A M, Townsend H G G, Allen A L, et al. 2008. Safety and immunogenicity of a live-attenuated auxotrophic candidate vaccine against the intracellular pathogen Rhodococcus equi. Vaccine, 26(7): 998–1009
Mayo M. 2002. A summary of taxonomic changes recently approved by ICTV. Archives of Virology, 147(8): 1655–1656
Namikoshi A, Wu Jinlu, Yamashita T, et al. 2004. Vaccination trialswith Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture, 229(1–4): 25–35
Ning Jianfang, Zhu Wei, Xu Jinping, et al. 2009. Oral delivery of DNA vaccine encoding VP28 against white spot syndrome virus in crayfish by attenuated Salmonella typhimurium. Vaccine, 27(7): 1127–1135
Okumura T. 2007. Effects of lipopolysaccharide on gene expression of antimicrobial peptides (penaeidins and crustin), serine proteinase and prophenoloxidase in haemocytes of the Pacific white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology, 22(1s-2): 68–76
Peng S E, Lo C F, Lin S C, et al. 2001. Performance of WSSV-infected and WSSV-negative Penaeus monodon postlarvae in culture ponds. Diseases of Aquatic Organisms, 46(3): 165–172
Rout N, Kumar S, Jaganmohan S, et al. 2007. DNA vaccines encoding viral envelope proteins confer protective immunity against WSSV in black tiger shrimp. Vaccine, 25(15): 2778–2786
Singh I S B, Manjusha M, Pai S S, et al. 2005. Fenneropenaeus indicus is protected from white spot disease by oral administration of inactivated white spot syndrome virus. Diseases of Aquatic Organisms, 66: 265–270
Söderhäll K, Cerenius L. 1992. Crustacean immunity. Annual Review of Fish Diseases, 2: 3–23
Söderhäll K, Häll L. 1984. Lipopolysaccharide-induced activation of prophenoloxidase activating system in crayfish haemocyte lysate. Biochimica et Biophysica Acta (BBA)-General Subjects, 797(1): 99–104
Syed Musthaq S, Madhan S, Sahul Hameed A, et al. 2009. Localization of VP28 on the baculovirus envelope and its immunogenicity against white spot syndrome virus in Penaeus monodon. Virology, 391(2): 315–324
Takahashi Y, Kondo M, Itami T, et al. 2000. Enhancement of disease resistance against penaeid acute viraemia and induction of virus-inactivating activity in haemolymph of kuruma shrimp, Penaeus japonicus, by oral administration of Pantoea agglomerans lipopolysaccharide (LPS). Fish & Shellfish Immunology, 10(6): 555–558
Tang Xuhua, Wu Jinlu, Sivaraman J, et al. 2007. Crystal structures of major envelope proteins VP26 and VP28 from white spot syndrome virus shed light on their evolutionary relationship. J Virol, 81(12): 6709–6717
Tsai M F, Kou G H, Liu H C, et al. 1999. Long-term presence of white spot syndrome virus (WSSV) in a cultivated shrimp population without disease outbreaks. Diseases of Aquatic Organisms, 38: 107–114
Tsai J M, Wang H C, Leu J H, et al. 2004. Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J Virol, 78(20): 11360–11370
van Hulten M C W, Witteveldt J, Peters S, et al. 2001. The white spot syndrome virus DNA genome sequence. Virology, 286(1): 7–22
van Hulten MCW, Witteveldt J, Snippe M, et al. 2001. White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp. Virology, 285(2): 228–233
Vaseeharan B, Prem Anand T, Murugan T, et al. 2006. Shrimp vaccination trials with the VP292 protein of white spot syndrome virus. Letters in Applied Microbiology, 43(2): 137–142
Venegas C A, Nonaka L, Mushiake K, et al. 2000. Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV). Diseases of Aquatic Organisms, 42(2): 83–89
Wang Wenxing, Luo Wantao, Song Qingyun, et al. 1996. Preliminary study on infection and transmission of Penaeus Chinensis explosive epidemic virus diseases in Expopalaemon Carinicauda (Holthuis) (in Chinese). Proceedings of the 2nd symposium on diseases of cultured penaeid shrimp. Qingdao: China Ocean University Press, 89–93
Wang Xingqiang, Yan Binlun, Ma Shen, et al. 2005. Study on the biology and cultural ecology of Expopalaemon Carinicauda. Shandong Fisheries (in Chinese), 22(8): 21–23
Witteveldt J, Cifuentes C C, Vlak JM, et al. 2004. Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. J Virol, 78(4): 2057–2061
Witteveldt J, Vlak J M, van Hulten M C W. 2004. Protection of Penaeus monodon against white spot syndrome virus using a WSSV subunit vaccine. Fish & Shellfish Immunology, 16(5): 571–579
Wu Wenlin, Wang Lei, Zhang Xiaobo. 2005. Identification of white spot syndrome virus (WSSV) envelope proteins involved in shrimp infection. Virology, 332(2): 578–583
Yang Feng, He Jun, Lin Xionghui, et al. 2001. Complete Genome Sequence of the Shrimp White Spot Bacilliform Virus. J Virol, 75(23): 11811–11820
Yao Qingxia, Qian Ping, Huang Qinfeng, et al. 2008. Comparison of immune responses to different foot-and-mouth disease genetically engineered vaccines in guinea pigs. Journal of Virological Methods, 147(1): 143–150
You Xinin, Su Yongquan, Mao Yong, et al. 2010. Effect of high water temperature on mortality, immune response and viral replication of WSSV-infected Marsupenaeus japonicus juveniles and adults. Aquaculture, 305: 133–137
Zhang Weiwei, Sun Kun, Cheng Shuang, et al. 2008. Characterization of DegQVh, a serine protease and a protective immunogen from a pathogenic vibrio harveyi strain. Appl Environ Microbiol, 74(20): 6254–6262
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The National Basic Research Program of China (973 Program) under contract No. 2012CB114403; General Program of National Natural Science Foundation of China to Dr. Fuhua Li under contract No. 31072203; National Hightech Research and Development Programunder contract Nos 2012AA10A404 and 2012AA092205; China Agriculture Research System under contract No. CARS-47.
Rights and permissions
About this article
Cite this article
Sun, Y., Li, F., Chi, Y. et al. Enhanced resistance of marine shrimp Exopalamon carincauda Holthuis to WSSV by injecting live VP28-recombinant bacteria. Acta Oceanol. Sin. 32, 52–58 (2013). https://doi.org/10.1007/s13131-013-0261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-013-0261-0