Abstract
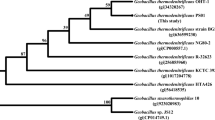
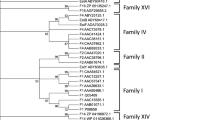
The gene (741 bp) encoding carboxylesterase from the thermophilic bacterium Geobacillus sp. ZHl was cloned and overexpressed in Escherichia coli. The purified recombinant protein presented a molecular mass of about 40 kDa by SDS-PAGE analysis. Enzyme assays using p-nitrophenyl esters with different acyl chain lengths as the substrates confirmed its esterase activity, yielding highest specific activity with p-nitrophenyl acetate. Among the p-nitrophenyl esters tested, the carboxylesterase presented preference for p-nitrophenyl caprylate, but hydrolyzed p-nitrophenyl butyrate more efficiently. When p-nitrophenyl butyrate was used as a substrate, the recombinant carboxylesterase exhibited highest activity at pH 8.0 and 60°C. Almost no decrease in esterase activity was observed at 60°C for 3 h, and over 40% of activity was still maintained after incubation at 90°C for 3 h. These results indicate that Geobacillus sp. ZH1 recombinant esterase was thermostable. The enzymatic activity was inhibited by the addition of phenylmethylsulfonyl fluoride, indicating that it contains serine residue, which plays a key role in the catalytic mechanism. Except SDS and xylene, this esterase showed stability toward other tested detergents and organic solvents. Cloning, expression, and biochemical characterization of Geobacillus sp. ZH1 carboxylesterase lay a good foundation for its structural characterization and industrial application.
Similar content being viewed by others
References
Almeida R V, Alquéres S M C, Larentis A L, et al. 2006. Cloning, expression, partial characterization and structural modeling of a novel esterase from Pyrococcus furiosus. Enzyme Microbial Technol, 39(5): 1128–1136
Altschul S F, Madden T L, Schäffer A A, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein databases search programs. Nucleic Acids Res, 25(17): 3389–3402
Arpigny J L, Jaeger K E. 1999. Bacterial lipolytic enzymes: classification and properties. Biochem J, 343(Pt 1): 177–183
Arpigny J L, Jendrossek D, Jaeger K E. 1998. A novel heat-stable lipolytic enzyme from Sulfolobus acidocaldarius DSM 639 displaying similarity to polyhydroxyalkanoate depolymerases. FEMS Microbiol Lett, 167(1): 69–73
Bornscheuer U T. 2002. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev, 26(1): 73–81
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—dye binding. Anal Biochem, 72: 248–254
Brod F C, Vernal J, Bertoldo J B, et al. 2010. Cloning, expression, purification, and characterization of a novel esterase from Lactobacillus plantarum. Mol Biotechnol, 44(3): 242–249
Charbonneau D M, Meddeb-Mouelhi F, Beauregard M. 2010. A novel thermostable carboxylesterase from Geobacillus thermodenitrificans: evidence for a new carboxylesterase family. J Biochem, 148(3): 299–308
du Plessis E M, Berger E, Stark T, et al. 2010. Characterization of a novel thermostable esterase from Thermus scotoductus SA-01: evidence of a new family of lipolytic esterases. Curr Microbiol, 60(4): 248–253
Ejima K, Liu Jian, Oshima Y, et al. 2004. Molecular cloning and characterization of a thermostable carboxylesterase from an archaeon, Sulfolobus shibatae DSM5389: non-linear kinetic behavior of a hormone-sensitive lipase family enzyme. J Biosci Bioeng, 98(6): 445–451
Fojan P, Jonson P H, Petersen M T, et al. 2000. What distinguishes an esterase from a lipase: a novel structural approach. Biochimie, 82(11): 1033–1041
Hasan F, Shah A A, Hameed A. 2006. Industrial applications of microbial lipases. Enzyme Microbial Technol, 39(2): 235–251
Hess M, Katzer M, Antranikian G. 2008. Extremely thermostable esterases from the thermoacidophilic euryarchaeon Picrophilus torridus. Extremophiles, 12(3): 351–364
Hotta Y, Ezaki S, Atomi H, et al. 2002. Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Appl Environ Microbiol, 68(8): 3925–3931
Jaeger K E, Dijkstra B W, Reetz M T. 1999. Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol, 53: 315–351
Jaeger K E, Reetz M T. 1998. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol, 16(9): 396–403
Kakugawa S, Fushinobu S, Wakagi T. 2007. Characterization of a thermostable carboxylesterase from the hyperthermophilic bacterium Thermotoga maritima. Appl Microbiol Biotechnol, 74(3): 585–591
Kim S B, Lee W, Ryu Y W. 2008. Cloning and characterization of thermostable esterase from Archaeoglobus fulgidus. J Microbiol, 46(1): 100–107
Laemmli U K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259): 680–685
Levisson M, van der Oost J, Kengen S W. 2007. Characterization and structural modeling of a new type of thermostable esterase from Thermotoga maritima. FEBS J, 274(11): 2832–2842
Manco G, Adinolfi E, Pisani F M, et al. 1998. Overexpression and properties of a new thermophilic and thermostable esterase from Bacillus acidocaldarius with sequence similarity to hormone-sensitive lipase subfamily. Biochem J, 332(Pt 1): 203–212
Manco G, Giosuè E, D’Auria S, et al. 2000. Cloning, overexpression, and properties of a new thermophilic and thermostable esterase with sequence similarity to hormone-sensitive lipase subfamily from the archaeon Archaeoglobus fulgidus. Arch Biochem Biophys, 373(1): 182–192
Morana A, Di Prizito N, Aurilia V, et al. 2002. A carboxylesterase from the hyperthermophilic archaeon Sulfolobus solfataricus: cloning of the gene, characterization of the protein. Gene, 283(1–2): 107–115
Ollis D L, Cheah E, Cygler M, et al. 1992. The alpha/beta hydrolase fold. Prot Eng, 5(3): 197–211
Oppenheimer C H, ZoBell C E. 1952. The growth and viability of sixty-three species of marine bacteria as influenced by hydrostatic pressure. J Mar Res, 11(1): 10–18
Park Y J, Choi S Y, Lee H B. 2006. A carboxylesterase from the thermoacidophilic archaeon Sulfolobus solfataricus P1; purification, characterization, and expression. Biochim Biophys Acta, 1760(5): 820–828
Sobek H, Görisch H. 1988. Purification and characterization of a heat-stable esterase from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochem J, 250(2): 453–458
Soliman N A, Knoll M, Abdel-Fattah Y R, et al. 2007. Molecular cloning and characterization of thermostable esterase and lipase from Geobacillus thermoleovorans YN isolated from desert soil in Egypt. Process Biochem, 42(7): 1090–1100
Stok J E, Huang Huazhang, Jones P D, et al. 2004. Identification, expression, and purification of a pyrethroid-hydrolyzing carboxylesterase from mouse liver microsomes. J Biol Chem, 279(28): 29863–29869
Sun Lei, Levisson M, Hendriks S, et al. 2007. Crystallization and preliminary crystallographic analysis of an esterase with a novel domain from the hyperthermophile Thermotoga maritima. Acta Crystallogr Sect F Struct Biol Cryst Commun, 63(Pt 9): 777–779
Suzuki Y, Miyamoto K, Ohta H. 2004. A novel thermostable esterase from the thermoacidophilic archaeon Sulfolobus tokodaii strain 7. FEMS Microbiol Lett, 236(1): 97–102
Tekedar H C, Şanlı-Mohamed G. 2011. Molecular cloning, over expression and characterization of thermoalkalophilic esterases isolated from Geobacillus sp. Extremophiles, 15(2): 203–211
Verger R. 1997. Interfacial activation of lipases: fact and artifacts. Trends Biotechnol, 15(1): 32–38
Vieille C, Zeikus G J. 2001. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev, 65(1): 1–43
Wang Baijing, Lu Dongmei, Gao Renjun, et al. 2004. A novel phospholipase A2/esterase from hyperthermophilic archaeon Aeropyrum pernix K1. Protein Expr Purif, 35(2): 199–205
Zhang Jian, Liu Jingfang, Zhou Jian, et al. 2003. Thermostable esterase from Thermoanaerobacter tengcongensis: high-level expression, purification and characterization. Biotechnol Lett, 25(17): 1463–1467
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Scientific Research Fund of Fujian Provincial Education Department, China under contact No. JA11153; the Natural Science Foundation of Fujian Province, China under contact Nos 2010J06012 and 2010J01261; the Foundation for Innovative Research Team of Jimei University, China under contact No. 2010A005.
Rights and permissions
About this article
Cite this article
Zhu, Y., Liu, G., Li, H. et al. Cloning and characterization of a thermostable carboxylesterase from inshore hot spring thermophile Geobacillus sp. ZH1. Acta Oceanol. Sin. 31, 117–126 (2012). https://doi.org/10.1007/s13131-012-0258-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-012-0258-0