Abstract
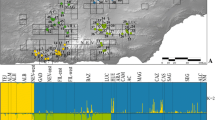
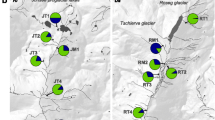
Water-dependent species inhabiting desert regions seem to be a contradiction in terms. Nevertheless, many species have evolved survival strategies for arid conditions. In Odonates (dragonflies and damselflies), both larvae and adults require very different and complex water-associated habitat conditions. The present study investigates the genetic diversity, population structure and dispersal patterns of a desert inhabiting odonate species, the Red-veined Dropwing dragonfly, Trithemis arteriosa. Eight populations from the arid Namibia and four population sites in the more tropical Kenya were compared by using nine microsatellite loci, one non-coding nuclear fragment and the mtDNA fragment ND1. Microsatellite analyses as well as the nuclear fragment reveal a high allelic diversity in all populations with almost no genetic sub-structuring. In contrast, ND1 sequence analyses show sub-structuring and—with two exceptions—only private haplotypes. The conflicting patterns of nuclear versus mitochondrial markers suggest a male-biased dispersal in this species. Results indicate that male dispersal is dependent on the environmental stability of the habitat, while females are philopatric. This life history adaptation would allow females to save energy for mating and oviposition in the demanding environment of a desert region. The results give direct insights into the dispersal pathways of a desert-inhabiting, strongly water dependent flying insect.



Similar content being viewed by others
References
Angelibert, S., & Giani, N. (2003). Dispersal characteristics of three odonate species in a patchy habitat. Ecography, 26(1), 13–20.
Artiss, T. (2004). Phylogeography of a facultatively migratory dragonfly, Libellula quadrimaculata (Odonata: Anisoptera). Hydrobiologia, 515(1–3), 225–234.
Avise, J. C., Arnold, J., Ball, R. M., Bermingham, E., Lamb, T., Neigel, J. E., Reeb, C. A., & Saunders, N. C. (1987). Intraspecific phylogeography—the mitochondrial-DNA bridge between population-genetics and systematics. Annual Review of Ecology and Systematics, 18, 489–522.
Barnard, P. (1998). Biological diversity in Namibia—a country study. Windhoek: Namibian National Biodiversity Task Force.
Beirinckx, K., Van Gossum, H., Lajeunesse, M. J., & Forbes, M. R. (2006). Sex biases in dispersal and philopatry: insights from a meta-analysis based on capture-mark-recapture studies of damselflies. Oikos, 113(3), 539–547.
Birky, C. W., Fuerst, P., & Maruyama, T. (1989). Organelle gene diversity under migration, mutation, and drift—equilibrium expectations, approach to equilibrium, effects of heteroplasmic cells, and comparison to nuclear genes. Genetics, 121(3), 613–627.
Bos, D. H., Gopurenko, D., Williams, R. N., & Dewoody, J. A. (2008). Inferring population history and demography using microsatellites, mitochondrial DNA, and major histocompatibility complex (MHC) genes. Evolution, 62(6), 1458–1468.
Bowler, D. E., & Benton, T. G. (2005). Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biological Reviews, 80(2), 205–225.
Cano, J. M., Makinen, H. S., & Merila, J. (2008). Genetic evidence for male-biased dispersal in the three-spined stickleback (Gasterosteus aculeatus). Molecular Ecology, 17(14), 3234–3242.
Clausnitzer, V. (2003). Dragonfly communities in coastal habitats of Kenya: indication of biotope quality and the need of conservation measures. Biodiversity and Conservation, 12(2), 333–356.
Clement, M., Posada, D., & Crandall, K. A. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9(10), 1657–1659.
Clobert, J., Danchin, E., Dhont, A. A., & Nichols, J. D. (2001). Dispersal. New York: Oxford University Press.
Corbet, P. (1999). Dragonflies—behaviour and ecology of Odonata. Colchester: Harley Books.
Cordero Rivera, A., & Stoks, R. (2008). Mark-recapture studies and demography. In A. Cordoba-Aguilar (Ed.), Dragonflies: model organism for ecological and evolutionary studies (pp. 7–20). Oxford: Oxford University Press.
Dallimer, M., Blackburn, C., Jones, P. J., & Pemberton, J. M. (2002). Genetic evidence for male biased dispersal in the red-billed quelea Quelea quelea. Molecular Ecology, 11(3), 529–533.
Damm, S., Dijkstra, K.-D. B., & Hadrys, H. (2010). Red drifters and dark residents: the phylogeny and ecology of a Plio-Pleistocene dragonfly radiation reflects Africa’s changing environment (Odonata, Libellulidae, Trithemis). Molecular Phylogenetics and Evolution, 54, 870–882.
Damm, S., & Hadrys, H. (2010). Dispersal strategies of desert inhabiting dragonflies—comparative population genetic study of five widely distributed species. In U. Schmiedel & N. Jürgens (Eds.), Biodiversity in southern Africa, Vol. 2: Patterns and processes at regional scale (pp. 167–174). Göttingen & Windhoek: Klaus Hess.
Damm, S., Schierwater, B., & Hadrys, H. (2010). An integrative approach to species discovery in odonates: from character-based DNA barcoding to ecology. Molecular Ecology, 19(18), 3881–3893.
Dijkstra, K. D. B. (2003). A review of the taxonomy of African Odonata: finding ways to better identification and biogreographic insight. Cimbebasia, 18, 191–206.
Dijkstra, K. D. B. (2007). The name-bearing types of Odonata held in the Natural History museum of Zimbabwe, with systematic notes on Afrotropical taxa. Part 1: Introduction and Anisoptera. International Journal of Odonatology, 10(1), 1–29.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14(8), 2611–2620.
Excoffier, L., Laval, G., & Schneider, S. (2005). Arlequin ver. 3.0: an intergrated software package for population genetic data analysis. Evolutionary Bioinformatics Online, 1, 47–50.
Excoffier, L., Smouse, P. E., & Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics, 131(2), 479–491.
Fincke, O. M., & Hadrys, H. (2001). Unpredictable offspring survivorship in the damselfly, Megaloprepus coerulatus, shapes parental behavior, constrains sexual selection, and challenges traditional fitness estimates. Evolution, 55(4), 762–772.
Freeland, J. R., May, M., Lodge, R., & Conrad, K. F. (2003). Genetic diversity and widespread haplotypes in a migratory dragonfly, the common green darner Anax junius. Ecological Entomology, 28(4), 413–421.
Fu, Y. X. (1997). Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics, 147(2), 915–925.
Gibbs, H. L., Dawson, R. J. G., & Hobson, K. A. (2000). Limited differentiation in microsatellite DNA variation among northern populations of the yellow warbler: evidence for male-biased gene flow? Molecular Ecology, 9(12), 2137–2147.
Giere, S., & Hadrys, H. (2006). Polymorphic microsatellite loci to study population dynamics in a dragonfly, the libellulid Trithemis arteriosa (Burmeister, 1839). Molecular Ecology Notes, 6(3), 933–935.
Goldstein, D. B., & Schlötterer, C. (Eds.). (1999). Microsatellites: Evolution and applications. Oxford: Oxford University Press.
Goudet, J. (2001). FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3.). Available from http://www.unil.ch/izea/softwares/fstat.html.
Griffin, M. (1998). The species diversity, distribution and conservation of Namibian mammals. Biodiversity and Conservation, 7(4), 483–494.
Groeneveld, L. F., Clausnitzer, V., & Hadrys, H. (2007). Convergent evolution of gigantism in damselflies of Africa and South America? Evidence from nuclear and mitochondrial sequence data. Molecular Phylogenetics and Evolution, 42(2), 339–346.
Gros, A., Hovestadt, T., & Poethke, H. J. (2008). Evolution of sex-biased dispersal: the role of sex-specific dispersal costs, demographic stochasticity, and inbreeding. Ecological Modelling, 219(1–2), 226–233.
Hadrys, H., Balick, M., & Schierwater, B. (1992). Applications of random amplified polymorphic DNA (RAPD) in molecular ecology. Molecular Ecology, 1(1), 55–63.
Hadrys, H., Schierwater, B., Dellaporta, S. L., Desalle, R., & Buss, L. W. (1993). Determination of paternity in dragonflies by random amplified polymorphic dna fingerprinting. Molecular Ecology, 2(2), 79–87.
Holland, R. A., Wikelski, M., & Wilcove, D. S. (2006). How and why do insects migrate? Science, 313(5788), 794–796.
Hurtado, L. A., Erez, T., Castrezana, S., & Markow, T. A. (2004). Contrasting population genetic patterns and evolutionary histories among sympatric Sonoran Desert cactophilic Drosophila. Molecular Ecology, 13(6), 1365–1375.
Jensen, J. L., Bohonak, A. J., & Kelley, S. T. (2005). Isolation by distance, web service. BMC Genetics, 6(1), 13.
Johansson, F., & Suhling, F. (2004). Behaviour and growth of dragonfly larvae along a permanent to temporary water habitat gradient. Ecological Entomology, 29(2), 196–202.
Kery, M., & Juillerat, L. (2004). Sex ratio estimation and survival analysis for Orthetrum coerulescens (Odonata, Libellulidae). Canadian Journal of Zoology-Revue Canadienne De Zoologie, 82(3), 399–406.
Lorenzen, E. D., Arctander, P., & Siegismund, H. R. (2008). High variation and very low differentiation in wide ranging plains zebra (Equus quagga) insights from mtDNA and microsatellites. Molecular Ecology, 17(12), 2812–2824.
Lukoschek, V., Waycott, M., & Keogh, J. S. (2008). Relative information content of polymorphic microsatellites and mitochondrial DNA for inferring dispersal and population genetic structure in the olive sea snake, Aipysurus laevis. Molecular Ecology, 17(13), 3062–3077.
Markow, T. A., & Castrezana, S. (2000). Dispersal in cactophilic Drosophila. Oikos, 89(2), 378–386.
Mccauley, S. (2005). Differential dispersal propensities between individuals in male Leucorrhina intacta (Odonata: Libellulidae). International Journal of Odonatology, 8(2), 223–232.
Mendelsohn, J., Jarvis, A., Roberts, C., Robertson, T. (2002). Atlas of Namibia—a portrait of the land and its people. Ministry of Environment and Tourism (Ed.). Cape Town: Philip.
Mesa, N. R., Mondragon, M. C., Soto, I. D., Parra, M. V., Duque, C., Ortiz-Barrientos, D., et al. (2000). Autosomal, mtDNA, and Y-chromosome diversity in Amerinds: pre- and post-Columbian patterns of gene flow in South America. American Journal of Human Genetics, 67(5), 1277–1286.
Müller, J., & Müller, K. (2003). QuickAlign: a new alignment editor. Plant Molecular Biology Reporter, 21(5).
Perrin, N., & Mazalov, V. (2000). Local competition, inbreeding, and the evolution of sex-biased dispersal. American Naturalist, 155(1), 116–127.
Pinhey, E. (1970). Monographic study of the genus Trithemis Brauer (Odonata:Libellulidae). Memoirs of the Entomological Society of Southern Africa, 11, 1–159.
Pritchard, J. K., Stephens, M., & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945–959.
Prugnolle, F., & De Meeus, T. (2002). Inferring sex-biased dispersal from population genetic tools: a review. Heredity, 88, 161–165.
Rach, J., Desalle, R., Sarkar, I. N., Schierwater, B., & Hadrys, H. (2008). Character-based DNA barcoding allows discrimination of genera, species and populations in Odonata. Proceedings of the Royal Society B-Biological Sciences, 275(1632), 237–247.
Raymond, M., & Rousset, F. (1995). Genepop (Version-1.2)—Population-genetics software for exact tests and ecumenicism. Journal of Heredity, 86(3), 248–249.
Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution, 43(1), 223–225.
Rousset, F. (1997). Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics, 145(4), 1219–1228.
Rousset, F. (2008). GENEPOP '007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources, 8(1), 103–106.
Rozas, J., Sanchez-Delbarrio, J. C., Messeguer, X., & Rozas, R. (2003). DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics, 19(18), 2496–2497.
Shmida, M. (1985). Biogeography of desert flora. In M. Evenari, I. Noy-Meir, & D. W. Goodall (Eds.), Hot deserts and arid shrublands (pp. 1–365). Amsterdam: Elsevier.
Simmons, R. E., Griffin, M., Griffin, R. E., Marais, E., & Kolberg, H. (1998). Endemism in Namibia: patterns, processes and predictions. Biodiversity and Conservation, 7(4), 513–530.
Sole, C. L., Bastos, A. D. S., & Scholtz, C. H. (2008). Intraspecific patterns of mitochondrial variation in natural population fragments of a localized desert dung beetle species, Pachysoma gariepinum (Coleoptera: Scarabaeidae). Journal of Heredity, 99(5), 464–475.
Stephens, M., Smith, N. J., & Donnelly, P. (2001). A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics, 68(4), 978–989.
Suhling, F., Jödicke, R., & Schneider, W. (2003). Odonata of African arid regions—are there desert species? Cimbebasia, 18, 207–224.
Suhling, F., Martens, A., & Marais, E. (2009). How to enter a desert—patterns of Odonata colonisation of arid Namibia. International Journal of Odonatology, 12(2), 287–308.
Suhling, F., Sahlen, G., Kasperski, J., & Gaedecke, D. (2005). Behavioural and life history traits in temporary and perennial waters: comparisons among three pairs of sibling dragonfly species. Oikos, 108(3), 609–617.
Suhling, F., Sahlen, G., Martens, A., Marais, E., & Schutte, C. (2006). Dragonfly assemblages in arid tropical environments: a case study from Western Namibia. Biodiversity and Conservation, 15(1), 311–332.
Tajima, F. (1989). Statistical-method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics, 123(3), 585–595.
Templeton, A. R., Crandall, K. A., & Sing, C. F. (1992). A cladistic-analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA-sequence data. 3. Cladogram estimation. Genetics, 132(2), 619–633.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24), 4876–4882.
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P. M., & Shipley, P. (2004). MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes, 4(3), 535–538.
Waples, R. S. (1998). Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species. Journal of Heredity, 89(5), 438–450.
Ward, D. (2009). The biology of deserts. New York: Oxford University Press.
Zhang, D. X., & Hewitt, G. M. (2003). Nuclear DNA analyses in genetic studies of populations: practice, problems and prospects. Molecular Ecology, 12(3), 563–584.
Acknowledgments
The work was supported by the program BIOTA South (S08) of the German Federal Ministry of Education and Research (BMBF). We are grateful to Viola Clausnitzer and Frank Suhling for providing us with specimens. We thank Eugene Marais (National Museum of Namibia) for his support, all collaborators for helping collect samples during our stay in Namibia, and two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is a contribution to the Festschrift for Michael L. May
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 15 kb)
Rights and permissions
About this article
Cite this article
Damm, S., Hadrys, H. A dragonfly in the desert: genetic pathways of the widespread Trithemis arteriosa (Odonata: Libellulidae) suggest male-biased dispersal. Org Divers Evol 12, 267–279 (2012). https://doi.org/10.1007/s13127-012-0079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-012-0079-1