Abstract
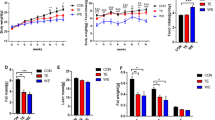
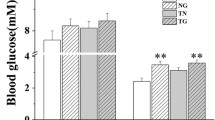
To identify factors that influence post-exercise muscle glycogen repletion, we compared the glycogen recovery after level running with downhill running, an experimental model of impaired post-exercise glycogen recovery. Male Institute of Cancer Research (ICR) mice performed endurance level running (no inclination) or downhill running (−5° inclination) on a treadmill. In Experiment 1, to determine whether these two types of exercise resulted in different post-exercise glycogen repletion patterns, tissues were harvested immediately post-exercise or 2 days post-exercise. Compared to the control (sedentary) group, level running induced significant glycogen supercompensation in the soleus muscle at 2 days post-exercise (p = 0.002). Downhill running did not induce glycogen supercompensation. In Experiment 2, mice were orally administered glucose 1 day post-exercise; this induced glycogen supercompensation in soleus and plantaris muscle only in the level running group (soleus: p = 0.005, plantaris: p = 0.003). There were significant positive main effects of level running compared to downhill running on the plasma insulin (p = 0.017) and C-peptide concentration (p = 0.011). There was no difference in the glucose transporter 4 level or the phosphorylated states of proteins related to insulin signaling and metabolism in skeletal muscle. The level running group showed significantly higher hexokinase 2 (HK2) protein content in both soleus (p = 0.046) and plantaris muscles (p =0.044) at 1 day after exercise compared to the downhill running group. Our findings suggest that post-exercise skeletal muscle glycogen repletion might be partly influenced by plasma insulin and skeletal muscle HK2 protein levels.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Aoi W, Naito Y, Tokuda H, Tanimura Y, Oya-Ito T, Yoshikawa T (2012) Exercise-induced muscle damage impairs insulin signaling pathway associated with IRS-1 oxidative modification. Physiol Res 61:81–88. https://doi.org/10.33549/physiolres.932239
Armstrong RB, Taylor CR (1993) Glycogen loss in rat muscles during locomotion on different inclines. J Exp Biol 176:135–144
Armstrong RB, Laughlin MH, Rome L, Taylor CR (1983) Metabolism of rats running up and down an incline. J Appl Physiol 55:518–521. https://doi.org/10.1152/jappl.1983.55.2.518
Armstrong RB, Ogilvie RW, Schwane JA (1983) Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54:80–93. https://doi.org/10.1152/jappl.1983.54.1.80
Asp S, Daugaard JR, Richter EA (1995) Eccentric exercise decreases glucose transporter GLUT4 protein in human skeletal muscle. J Physiol 482:705–712. https://doi.org/10.1113/jphysiol.1995.sp020553
Asp S, Kristiansen S, Richter EA (1995) Eccentric muscle damage transiently decreases rat skeletal muscle GLUT-4 protein. J Appl Physiol 79:1338–1345. https://doi.org/10.1152/jappl.1995.79.4.1338
Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA (1996) Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol 494(Pt 3):891–898. https://doi.org/10.1113/jphysiol.1996.sp021541
Asp S, Rohde T, Richter EA (1997) Impaired muscle glycogen resynthesis after a marathon is not caused by decreased muscle GLUT-4 content. J Appl Physiol 83:14821485. https://doi.org/10.1152/jappl.1997.83.5.1482
Asp S, Daugaard JR, Rohde T, Adamo K, Graham T (1999) Muscle glycogen accumulation after a marathon: roles of fiber type and pro- and macroglycogen. J Appl Physiol 86:474–478. https://doi.org/10.1152/jappl.1999.86.2.474
Bergström J, Hultman E (1966) Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210:309–310. https://doi.org/10.1038/210309a0
Burke LM, van Loon LJC, Hawley JA (2017) Postexercise muscle glycogen resynthesis in humans. J Appl Physiol 122:1055–1067. https://doi.org/10.1152/japplphysiol.00860.2016
Costill DL, Pascoe DD, Fink WJ, Robergs RA, Barr SI, Pearson D (1990) Impaired muscle glycogen resynthesis after eccentric exercise. J Appl Physiol 69:46–50. https://doi.org/10.1152/jappl.1990.69.1.46
Del Aguila LF, Krishnan RK, Ulbrecht JS, Farrell PA, Correll PH, Lang CH, Zierath JR, Kirwan JP (2000) Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab 279:E206–E212. https://doi.org/10.1152/ajpendo.2000.279.1.E206
Derave W, Lund S, Holman GD, Wojtaszewski J, Pedersen O, Richter EA (1999) Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen content. Am J Phys 277:E1103–E1110. https://doi.org/10.1152/ajpendo.1999.277.6.E1103
Doyle JA, Sherman WM, Strauss RL (1993) Effects of eccentric and concentric exercise on muscle glycogen replenishment. J Appl Physiol 74:1848–1855. https://doi.org/10.1152/jappl.1993.74.4.1848
Flores-Opazo M, McGee SL, Hargreaves M (2020) Exercise and GLUT4. Exerc Sport Sci Rev 48:110–118. https://doi.org/10.1249/JES.0000000000000224
Hansen PA, Nolte LA, Chen MM, Holloszy JO (1998) Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85:1218–1222. https://doi.org/10.1152/jappl.1998.85.4.1218
Hawley JA, Leckey JJ (2015) Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med 45(Suppl 1):S5–S12. https://doi.org/10.1007/s40279-015-0400-1
Hickner RC, Fisher JS, Hansen PA, Racette SB, Mier CM, Turner MJ, Holloszy JO (1997) Muscle glycogen accumulation after endurance exercise in trained and untrained individuals. J Appl Physiol 83:897–903. https://doi.org/10.1152/jappl.1997.83.3.897
Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K, Færgeman NJ, Havelund JF, Parker BL, James DE, Kiens B, Richter EA, Jensen J, Wojtaszewski JFP (2018) Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol Metab 16:24–34. https://doi.org/10.1016/j.molmet.2018.07.001
Irimia JM, Rovira J, Nielsen JN, Guerrero M, Wojtaszewski JFP, Cussó R (2012) Hexokinase 2, glycogen synthase and phosphorylase play a key role in muscle glycogen supercompensation. PLoS One 7:e42453. https://doi.org/10.1371/journal.pone.0042453
Krishnan RK, Hernandez JM, Williamson DL, O’Gorman DJ, Evans WJ, Kirwan JP (1998) Age-related differences in the pancreatic beta-cell response to hyperglycemia after eccentric exercise. Am J Phys 275:E463–E470. https://doi.org/10.1152/ajpendo.1998.275.3.E463
Kristiansen S, Jones J, Handberg A, Dohm DL, Richher EA (1997) Eccentric contractions decrease glucose transporter transcription rate, mRNA, and protein in skeletal muscle. Am J Phys 272:C1734–C1738. https://doi.org/10.1152/ajpcell.1997.272.5.C1734
Krustrup P, Ortenblad N, Nielsen J, Nybo L, Gunnarsson TP, Iaia FM, Madsen K, Stephens F, Greenhaff P, Bangsbo J (2011) Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Eur J Appl Physiol 111:2987–2995. https://doi.org/10.1007/s00421-011-1919-y
Lo S, Russell JC, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol 28:234–236. https://doi.org/10.1152/jappl.1970.28.2.234
Mandarino LJ, Printz RL, Cusi KA, Kinchington P, O’Doherty RM, Osawa H, Sewell C, Consoli A, Granner DK, DeFronzo RA (1995) Regulation of hexokinase II and glycogen synthase mRNA, protein, and activity in human muscle. Am J Phys 269:E701–E708. https://doi.org/10.1152/ajpendo.1995.269.4.E701
Mehta VK, Hao W, Brooks-Worrell BM, Palmer JP (1994) Low-dose interleukin 1 and tumor necrosis factor individually stimulate insulin release but in combination cause suppression. Eur J Endocrinol 130:208–214. https://doi.org/10.1530/eje.0.1300208
Nakatani A, Han DH, Hansen PA, Nolte LA, Host HH, Hickner RC, Holloszy JO (1997) Effect of endurance exercise training on muscle glycogen supercompensation in rats. J Appl Physiol 82:711–715. https://doi.org/10.1152/jappl.1997.82.2.711
Overmyer KA, Thonusin C, Qi NR, Burant CF, Evans CR (2015) Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: studies in a C57BL/6J mouse model. PLoS One 10:e0117232. https://doi.org/10.1371/journal.pone.0117232
Roberts DJ, Miyamoto S (2015) Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ 22:248–257. https://doi.org/10.1038/cdd.2014.173
Stephens FB, Norton L, Jewell K, Chokkalingam K, Parr T, Tsintzas K (2010) Basal and insulin-stimulated pyruvate dehydrogenase complex activation, glycogen synthesis and metabolic gene expression in human skeletal muscle the day after a single bout of exercise. Exp Physiol 95:808–818. https://doi.org/10.1113/expphysiol.2009.051367
Takahashi Y, Matsunaga Y, Banjo M, Takahashi K, Sato Y, Seike K, Nakano S, Hatta H (2019) Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and its Related Signaling Pathways in Mouse Skeletal Muscle. Nutrients 11. https://doi.org/10.3390/nu11112555
Tanaka K, Kawano T, Tomino T, Kawano H, Okada T, Oshita S, Takahashi A, Nakaya Y (2009) Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology 111:1044–1051. https://doi.org/10.1097/ALN.0b013e3181bbcb0d
Villar-Palasí C, Guinovart JJ (1997) The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J 11:544–558. https://doi.org/10.1096/fasebj.11.7.9212078
Vogt C, Ardehali H, Iozzo P, Yki-Järvinen H, Koval J, Maezono K, Pendergrass M, Printz R, Granner D, DeFronzo R, Mandarino L (2000) Regulation of hexokinase II expression in human skeletal muscle in vivo. Metabolism 49:814–818. https://doi.org/10.1053/meta.2000.6245
Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS (2011) The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 214:254–262. https://doi.org/10.1242/jeb.048041
Weber FE, Pette D (1990) Changes in free and bound forms and total amount of hexokinase isozyme II of rat muscle in response to contractile activity. Eur J Biochem 191:85–90. https://doi.org/10.1111/j.1432-1033.1990.tb19096.x
Widrick JJ, Costill DL, McConell GK, Anderson DE, Pearson DR, Zachwieja JJ (1992) Time course of glycogen accumulation after eccentric exercise. J Appl Physiol 72:1999–2004. https://doi.org/10.1152/jappl.1992.72.5.1999
Windeløv JA, Pedersen J, Holst JJ (2016) Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Phys Rep 4:e12824. https://doi.org/10.14814/phy2.12824
Wojtaszewski JF, Hansen BF, Gade, Kiens B, Markuns JF, Goodyear LJ, Richter EA (2000) Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49:325–331. https://doi.org/10.2337/diabetes.49.3.325
Zachwieja JJ, Costill DL, Pascoe DD, Robergs RA, Fink WJ (1991) Influence of muscle glycogen depletion on the rate of resynthesis. Med Sci Sports Exerc 23:44–48. https://doi.org/10.1249/00005768-199101000-00008
Zawadzki KM, Yaspelkis BB 3rd, Ivy JL (1992) Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J Appl Physiol 72:1854–1859. https://doi.org/10.1152/jappl.1992.72.5.1854
Funding
This research was financially supported by Grant-in-Aid for Young Scientists (18K17853) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Conceptualization, YT, JS, and HH; data curation, YT, JS, JY, YM, MB, RS, and TS; formal analysis, YT, JS, and JY; data interpretation; YT, JS, YM, YN, and HH; writing—original draft preparation, YT, JS, and HH. All authors reviewed and then approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All procedures involving animals were conducted in accordance with the ethical standards of the Committee on Animal Care and Use, The University of Tokyo; all experimental protocols were approved by this committee (approval no. 24-4).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• We compared two running models to find factors stimulating glycogen recovery.
• Greater glycogen recovery was collateral with higher muscle HK2 and plasma insulin.
• Greater glycogen recovery was not accompanied with activated insulin signaling.
Rights and permissions
About this article
Cite this article
Takahashi, Y., Sarkar, J., Yamada, J. et al. Enhanced skeletal muscle glycogen repletion after endurance exercise is associated with higher plasma insulin and skeletal muscle hexokinase 2 protein levels in mice: comparison of level running and downhill running model. J Physiol Biochem 77, 469–480 (2021). https://doi.org/10.1007/s13105-021-00806-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00806-z