Abstract
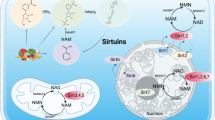
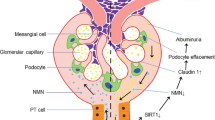
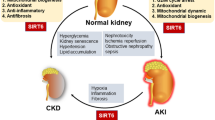
Sirtuins, silent information regulator 2 (Sir 2) proteins, belong to the family of NAD+-dependent enzymes with deacetylase or mono-ADP-ribosyltransferase activity. These enzymes are responsible for processes of DNA repair or recombination, chromosomal stability and gene transcription. In mammals, sirtuins occur in seven varieties, from 1 to 7 (SIRT1–SIRT7), differing among themselves with location. SIRT1, the best known variety, exerts its effects on proteins via NAD+ coenzymes, being thus associated with cellular energetic metabolism and the ‘red–ox’ state. Its deficits are, among others, concomitant with stressful situations and associated with pathophysiologies of many medical conditions, including diabetes mellitus, cardiovascular diseases, neurodegenerative syndromes and kidney diseases. In kidney disorders, it promotes (stimulates) the survival of cells in an affected kidney by modulating their responses to various stress stimuli, takes part in arterial blood pressure control, protects against cellular apoptosis in renal tubules by catalase induction and triggers autophagy. More and more available in vitro and in vivo data indicate SIRT1 activity to be oriented, among others, towards nephroprotection. Thus, SIRT1 may become a novel element in the therapy of age-related renal diseases, including diabetic nephropathy.

Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting-enzyme
- Atg5:
-
Autophagy protein 5
- Atg7:
-
Autophagy protein 7
- ATP:
-
Adenosine-5'-triphosphate
- COX2 :
-
Cyclooxygenase-II enzyme
- CR:
-
Caloric restriction
- DNA:
-
Deoxyribonucleic acid
- Dot1:
-
Disruptor of telomeric silencing-1
- ENaC:
-
Epithelial sodium channel
- ERK:
-
Extracellular signal-regulated kinases
- FOXO3 :
-
Forkhead box O3
- FXR:
-
farnesoid X receptor
- HIF-2a:
-
hypoxia-inducible factor
- Ku70:
-
Protein that, in humans, is encoded by the XRCC6 gene
- LC3:
-
protein light chain 3
- LXR:
-
liver X receptor
- Lys310:
-
lysine 310
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NAM:
-
Generating nicotinamide
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitrogen oxide
- PGE2 :
-
prostaglandin E2
- AT1R:
-
angiotensin II receptor, type 1
- SIRT:
-
Silent information regulator
- Smad3:
-
Mothers against decapentaplegic homolog 3
- SREBP:
-
sterol regulatory element binding proteins
- TGF-β1:
-
Transforming growth factor beta
- UCP2:
-
uncoupling protein 2
References
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J (2007) SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6(6):759–767
Cohen HY, Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasle TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325(5937):201–204
Hao CH, Haase VH (2010) Sirtuins and their relevance to the kidney. J Am Soc Nephrol 21(10):1620–1627
Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H (2008) Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 372(1):51–56
Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M (2010) Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285(17):13045–13056
He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC (2010) Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120(4):1056–1068
Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D (2013) Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci 124(3):153–164
Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, Koya D (2011) Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res 2011:908185
Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120(4):1043–1055
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105(9):3374–3379
Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ (2010) Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 177(3):1065–1071
Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW (2010) Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol Cell 38(6):864–878
McCay CM, Crowell MF, Maynard LA (1989) The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr 5(3):155–171
Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, Sunagawa K (2008) SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 28(7):1263–1269
Stępień A, Izdebska M, Grzanka A (2007) The types of cell death. Postepy Hig Med Dosw 61(9):420–428
Yamamoto H, Schoonjans K, Auwerx J (2007) Sirtuin functions in health and disease. Mol Endocrinol 21(8):1745–1755
Yu J, Auwerx J (2009) The role of sirtuins in the control of metabolic homeostasis integrative physiology. Ann NY Acad Sci 1173(1):E10–E19
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polak-Jonkisz, D., Laszki-Szcząchor, K., Rehan, L. et al. Nephroprotective action of sirtuin 1 (SIRT1). J Physiol Biochem 69, 957–961 (2013). https://doi.org/10.1007/s13105-013-0268-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0268-1