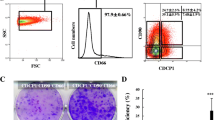
Abstract
Hepatocyte transplantation is considered a promising therapy for patients with liver diseases. Induced pluripotent stem cells (iPSCs) are an unlimited source for the generation of functional hepatocytes. While several protocols that direct the differentiation of iPSCs into hepatocyte-like cells have already been reported, the liver engraftment potential of iPSC progeny obtained at each step of hepatic differentiation has not yet been thoroughly investigated. In this study, we present an efficient strategy to differentiate mouse iPSCs into hepatocyte-like cells and evaluate their liver engraftment potential at different time points of the protocol (5, 10, 15, and 20 days of differentiation). iPSCs were differentiated in the presence of cytokines, growth factors, and small molecules to finally generate hepatocyte-like cells. These iPSC-derived hepatocyte-like cells exhibited hepatocyte-associated functions, such as albumin secretion and urea synthesis. When we transplanted iPSC progeny into the spleen, we found that 15- and 20-day iPSC progeny engrafted into the livers and further acquired hepatocyte morphology. In contrast, 5- and 10-day iPSC progeny were also able to engraft but did not generate hepatocyte-like cells in vivo. Our data may aid in improving current protocols geared towards the use of iPSCs as a new source of liver-targeted cell therapies.






Similar content being viewed by others
References
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Izpisua Belmonte JC (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26:1276h–1284h
Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321:699–702
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5:111–123
D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541
Dhawan A, Strom SC, Sokal E, Fox IJ (2010) Human hepatocyte transplantation. Methods Mol Biol 640:525–534
Elaut G, Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, Rogiers V (2006) Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab 7:629–660
Gai H, Nguyen DM, Moon YJ, Aguila JR, Fink LM, Ward DC & Ma Y (2010) Generation of murine hepatic lineage cells from induced pluripotent stem cells. Differentiation; research in biological diversity 79:171–181
Guguen-Guillouzo C, Guillouzo A (2010) General review on in vitro hepatocyte models and their applications. Methods Mol Biol 640:1–40
Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N (2001) Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 497:15–19
Hay DC, Zhao DB, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, Cui W (2008) Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26:894–902
Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467:285–290
Kwon GS, Fraser ST, Eakin GS, Mangano M, Isern J, Sahr KE, Hadjantonakis AK, Baron MH (2006) Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev Dyn 235:2549–2558
Kwon GS, Hadjantonakis AK (2009) Transthyretin mouse transgenes direct RFP expression or Cre-mediated recombination throughout the visceral endoderm. Genesis 47:447–455
Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY (2011) In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med 3:82ra39
Morrison GM, Oikonomopoulou I, Migueles RP, Soneji S, Livigni A, Enver T, Brickman JM (2008) Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell 3:402–415
Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101–106
Nakamura N, Saeki K, Mitsumoto M, Matsuyama S, Nishio M, Saeki K, Hasegawa M, Miyagawa Y, Ohkita H, Kiyokawa N, Toyoda M, Akutsu H, Umezawa A, Yuo A (2012) Feeder-Free and Serum-Free Production of Hepatocytes, Cholangiocytes, and Their Proliferating Progenitors from Human Pluripotent Stem Cells: Application to Liver-Specific Functional and Cytotoxic Assays. Cell Reprogram 14:171–185
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Sancho-Bru P, Roelandt P, Narain N, Pauwelyn K, Notelaers T, Shimizu T, Ott M, Verfaillie C (2011) Directed differentiation of murine-induced pluripotent stem cells to functional hepatocyte-like cells. J Hepatol 54:98–107
Snykers S, De Kock J, Rogiers V, Vanhaecke T (2009) In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells 27:577–605
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H (2009) Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res 19:1233–1242
Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, Tanaka K, Narushima M, Miki A, Ueda T, Jun HS, Yoon JW, Lebkowski J, Tanaka N, Fox IJ (2006) Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol 24:1412–1419
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T, Nishikawa S (2005) Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol 23:1542–1550
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zorn AM, Wells JM (2009) Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25:221–251
Acknowledgments
We thank Dr. Miguel Barajas for providing us retroviral plasmids for reprogramming. We thank Dr. Angelo Porciuncula for not only correcting the English but also for some valuable comments. This work is supported by Foundation for Applied Medical Research (FIMA), University of Navarra, Spain.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
a Characterisation of iPSCs by immunofluorescence. Images show the immunodetection of Oct4 (×20), Nanog (×20), and SSEA-1 (×20) in iPSC clones (clones 1.38 and 1.39). The red signal indicates the stem cell markers Oct4, Nanog, and SSEA-1 in iPSCs. The blue signal indicates the nuclei of iPSCs and feeder cells. Immunofluorescence without primary antibody was used as the negative control (named as control). No stem cell markers were detected in feeder cells. b Detection of alkaline phosphatase in iPSC clones 1.38 and 1.39. Images of alkaline phosphatase-stained iPSC clones grown on feeder cells and feeder cells alone are shown (magnification, ×10). The violet stain indicates the presence of alkaline phosphatase in the iPSC clones. Feeder cells were used as the negative control. c qPCR analysis of c-Myc levels in primary mouse tail tip fibroblasts and hepatocytes. The gene expression values of c-Myc in mouse tail tip fibroblasts are set to 1. (TIFF 10821 kb)
Supplementary Fig. 2
qPCR expression analysis of hepatic markers. iPSCs differentiated for 5, 10, 15, and 20 days were analysed for the expression of Arginase-1, Hnf1α, Hnf1β, C/EBPα, and C/EBPβ. The CT values of all genes were normalised to the CT values of β-actin. The y-axis represents the fold change of gene expression compared with undifferentiated iPSCs. One-way ANOVA test was used for statistical analysis (*p < 0.05; **p < 0.01; ***p < 0.001). Error bars represent the standard deviation. The data represent the average values of triplicates of the same experiment. (TIFF 10820 kb)
Supplementary Table 1
Primer sequences used in this study. (TIFF 10,801 kb)
Supplementary Table 2
Increased gene expression of hepatic markers in 20-day iPSC progeny and mouse hepatocytes compared with the observed in undifferentiated iPSCs. (TIFF 10,818 kb)
Rights and permissions
About this article
Cite this article
Balasiddaiah, A., Moreno, D., Guembe, L. et al. Hepatic differentiation of mouse iPS cells and analysis of liver engraftment potential of multistage iPS progeny. J Physiol Biochem 69, 835–845 (2013). https://doi.org/10.1007/s13105-013-0260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0260-9