Abstract
An 86-year-old patient was admitted to the ICU with multiple trauma injury following a fall down ten stairs. Shortly after ICU admission the patient went into cardiac arrest and resuscitation efforts, including cardiopulmonary resuscitation (CPR), were initiated. Transesophageal echocardiogram was performed to guide therapy and, after regaining heart rhythm and blood pressure, the echocardiogram exam demonstrated critical aortic stenosis and type-B aortic dissection. The aortic dissection was not present on initial chest computerized tomography done on admission to the hospital. The aortic dissection appears to have resulted from the CPR performed to revive the patient.
Similar content being viewed by others
Introduction
An 86-year-old female was transferred to our hospital after falling down ten stairs. Past medical history included Alzheimer dementia, hypertension, osteoarthritis, gastroesophageal reflux disease, hiatal hernia and hypercholesterolemia. The patient’s injuries included right eighth through tenth rib fractures and abdominal distension. The patient was admitted to the intensive care unit (ICU) after an episode of hypotension in the emergency department. Blood pressure improved with fluid therapy. Ninety minutes after ICU admission, the patient became unresponsive and went into pulseless electrical activity (PEA). Advanced Cardiac Life Support (ACLS) protocol was initiated; external cardiac massage (CPR) was started, the patient was intubated and resuscitative medications were given. A right-sided chest tube was placed with immediate drainage of 400 ml of blood. During the resuscitation efforts, a transesophageal echocardiogram (TEE) was performed to direct management as a routine practice in such circumstances in our ICU. After half an hour of resuscitative efforts the patient regained pulse and blood pressure.
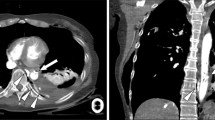
Following return of spontaneous heart rhythm and blood pressure, a complete TEE exam demonstrated severe aortic stenosis with an aortic valve area on 0.8 cm2. Hemodynamic management was modified based on the new echocardiographic findings. While completing the TEE exam, a type B aortic dissection was diagnosed in the descending aorta (Fig. 1). The patient had no previous evidence of aortic dissection on a computerized tomographic angiogram performed on admission to the hospital a few hours earlier (Fig. 2). The patient was taken to the operating room for exploratory laparotomy and remained hemodynamically stable throughout her hospitalization. Due to poor neurological status the patient’s family elected to withdrawal care and the patient expired 9 days after her admission to the hospital.
Discussion
To our knowledge this is the first description of CPR-induced type-B aortic dissection. The recent American Heart Association CPR guidelines recommend: “to give ‘effective’ chest compressions, ‘push hard and push fast…’”[1]. Such aggressive resuscitation attempts require aggressive manipulation of a patient, and may lead to complications. These resuscitation-related injuries include damage to the chest, airway and abdomen. The most frequent bony injuries seen as a result of CPR in adults are rib (frequency of 13–97%) and sternal (frequency 1–43%) fractures [2]. Fractured ribs can cause pericardial tamponade, injuries of the myocardium, pericardium, pleura, diaphragm and pneumothoraces as well as hemothoraces. In such cases, success of resuscitation efforts is negatively affected [3].
Although visceral structures are affected less than thoracic structures from resuscitation-related trauma, upper abdominal organs such as liver, stomach and spleen might be injured as well [4–6]. These injuries result mainly from fractured ribs or sternum which can lead to visceral perforation and rupture during CPR.
Vascular injuries as a result of CPR are very rare. Klintschar et al. [5] reported a case of an 84-year-old female following 3 min of conventional CPR and 15 min of active compression–decompression device, which resulted in an extensive cardiac injury that included a series of rib fractures, a transverse fracture of the sternum, and rupture of the pericardial sac, the right ventricle, both atria and lacerations of the ascending aorta. In this case, however, the trauma from CPR was not isolated to the aorta and included heart structures as well. Isolated aortic rupture from CPR is rare, and has been described in the literature with a frequency of 1% [7].
Unlike ventricular fibrillation and pulseless ventricular tachycardia where the pattern and rhythm of electrical activity is the focus of treatment rather than the underlying cause, PEA and asystole are corrected by addressing the underlying cause [8]. Ultrasound is a diagnostic tool with increasing applications and use in emergency situations [9]. Echocardiography can help with rapid diagnosis of etiology of PEA arrest and asystole, and guide therapy during these crucial moments. In our case it was critical for the diagnosis of an unknown severe aortic stenosis and helped us guide appropriate therapy. Finally, without echocardiography, the diagnosis of type B aortic dissection would have not been made.
Conclusion
Our case expands the knowledge of complications from external cardiac massage. In addition to injuries to the airway, chest and abdomen, we have shown that blood vessels can be injured, specifically the aorta. In addition, our case demonstrates the value of incorporating echocardiography in the management algorithm of patients in PEA arrest.
References
American Heart Association (2005) Guidelines for CPR and ECC—Part 4: adult basic life support. Circulation 112:IV-19–IV-3
Hoke RS, Chamberlain D (2004) Skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 63:327–338
Sokolove PE, Willis-Shore J, Panacek EA (2002) Exsanguination due to right ventricular rupture during closed-chest cardiopulmonary resuscitation. J Emerg Med 23:161–164
Noffsinger AE, Blisard KS, Balko MG (1991) Cardiac laceration and pericardial tamponade due to cardiopulmonary resuscitation after myocardial infarction. J Forensic Sci 36:1760–1764
Klintschar M, Darok M, Radner H (1998) Massive injury to the heart after attempted active compression–decompression cardiopulmonary resuscitation. Int J Legal Med 111:93–96
Meron G, Kurkciyan I, Sterz F et al (2007) Cardiopulmonary resuscitation-associated major liver injury. Resuscitation 75:445–453
Bode G, Joachim H (1987) Zur Differntialdiagnose von Unfall- und Reanimationstraumen. Z Rechtsmed 98:19–32
Cummins RO (ed) (2002) ACLS provider manual. American Heart Association, Dallas, pp 97–98
MacCarthy P, Worrall A, McCCarthy G et al (2002) The use of transthoracic echocardiogram to guide thrombolytic therapy during cardiac arrest due to massive pulmonary embolism. Emerg Med J 19:178–179
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oren-Grinberg, A., Shahul, S. & Sarge, T. Dissection of the thoracic aorta following cardiopulmonary resuscitation. Crit Ultrasound J 3, 25–27 (2011). https://doi.org/10.1007/s13089-011-0056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13089-011-0056-5