Abstract
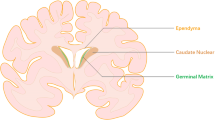
Germinal matrix hemorrhage (GMH) can be a fatal condition responsible for the death of 1.7% of all neonates in the USA. The majority of GMH survivors develop long-term sequalae with debilitating comorbidities. Higher grade GMH is associated with higher mortality rates and higher prevalence of comorbidities. The pathophysiology of GMH can be broken down into two main titles: faulty hemodynamic autoregulation and structural weakness at the level of tissues and cells. Prematurity is the most significant risk factor for GMH, and it predisposes to both major pathophysiological mechanisms of the condition. Secondary brain injury is an important determinant of survival and comorbidities following GMH. Mechanisms of brain injury secondary to GMH include apoptosis, necrosis, neuroinflammation, and oxidative stress. This review will have a special focus on the mechanisms of oxidative stress following GMH, including but not limited to inflammation, mitochondrial reactive oxygen species, glutamate toxicity, and hemoglobin metabolic products. In addition, this review will explore treatment options of GMH, especially targeted therapy.

Similar content being viewed by others
Abbreviations
- Bcl-2/Bax:
-
B cell lymphoma protein 2/Bcl-2-associated X
- H2O2 :
-
Hydrogen peroxide
- IVH:
-
Intraventricular hemorrhage
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NLRP3:
-
Nucleotide-binding domain-like receptor protein 3
- NMDA:
-
N-methyl-d-aspartate
- NMDAR:
-
NMDA receptors
- NO:
-
Nitric oxide
- NOS:
-
Neuronal nitric oxide synthase
- NOX:
-
NADPH oxidase
- ONOO- :
-
Peroxynitrite
- O2 - :
-
Superoxide
- PPARγ:
-
Proliferator-activated receptor-gamma
- ROS:
-
Reactive oxygen species
- TH:
-
Therapeutic hypothermia
- Wnt:
-
Wingless/integrated
- OH:
-
Hydroxyl radical
References
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. https://doi.org/10.1016/j.nbd.2003.12.016.
Brouwer AJ, Groenendaal F, Benders MJ, de Vries LS. Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Neonatology. 2014;106:296–303. https://doi.org/10.1159/000365127.
Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 2015;136:1132–43. https://doi.org/10.1542/peds.2015-0944.
O'Shea TM, Allred EN, Kuban KC, Hirtz D, Specter B, Durfee S, Paneth N, Leviton A. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol. 2012;27:22–9. https://doi.org/10.1177/0883073811424462.
Song J, Nilsson G, Xu Y, Zelco A, Rocha-Ferreira E, Wang Y, Zhang X, Zhang S, Ek J, Hagberg H, et al. Temporal brain transcriptome analysis reveals key pathological events after germinal matrix hemorrhage in neonatal rats. J Cereb Blood Flow Metab. 2022;42:1632–49. https://doi.org/10.1177/0271678x221098811.
Tan AP, Svrckova P, Cowan F, Chong WK, Mankad K. Intracranial hemorrhage in neonates: a review of etiologies, patterns and predicted clinical outcomes. Eur J Paediatr Neurol. 2018;22:690–717. https://doi.org/10.1016/j.ejpn.2018.04.008.
Klebe D, McBride D, Krafft PR, Flores JJ, Tang J, Zhang JH. Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: established mechanisms and proposed pathways. J Neurosci Res. 2020;98:105–20. https://doi.org/10.1002/jnr.24394.
Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2004;56:117–24. https://doi.org/10.1203/01.Pdr.0000130472.30874.Ff.
Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–85. https://doi.org/10.1038/nm1558.
Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41:47–67. https://doi.org/10.1016/j.clp.2013.09.007.
Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M. The premature brain: developmental and lesional anatomy. Neuroradiology. 2013;55(Suppl 2):23–40. https://doi.org/10.1007/s00234-013-1231-0.
Takashima S, Tanaka K. Microangiography and vascular permeability of the subependymal matrix in the premature infant. Can J Neurol Sci. 1978;5:45–50.
Cuestas E, Bas J, Pautasso J. Sex differences in intraventricular hemorrhage rates among very low birth weight newborns. Gend Med. 2009;6:376–82. https://doi.org/10.1016/j.genm.2009.06.001.
Rosenkrantz TS, Hussain Z, Fitch RH. Sex differences in brain injury and repair in newborn infants: clinical evidence and biological mechanisms. Front Pediatr. 2019;7:211. https://doi.org/10.3389/fped.2019.00211.
Baenziger O, Jaggi JL, Mueller AC, Morales CG, Lipp HP, Lipp AE, Duc G, Bucher HU. Cerebral blood flow in preterm infants affected by sex, mechanical ventilation, and intrauterine growth. Pediatr Neurol. 1994;11:319–24. https://doi.org/10.1016/0887-8994(94)90009-4.
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. https://doi.org/10.1203/PDR.0b013e3181c1b176.
Parodi A, Govaert P, Horsch S, Bravo MC, Ramenghi LA, Agut T, Alarcon A, Arena R, Bartocci M, Bravo M, et al. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr Res. 2020;87:13–24. https://doi.org/10.1038/s41390-020-0780-2.
Ballabh P, de Vries LS. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat Rev Neurol. 2021;17:199–214. https://doi.org/10.1038/s41582-020-00447-8.
Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21:1111–22. https://doi.org/10.1080/14728222.2017.1397628.
Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–8. https://doi.org/10.1161/strokeaha.108.535419.
Romantsik O, Bruschettini M, Ley D. Intraventricular hemorrhage and white matter injury in preclinical and clinical studies. Neoreviews. 2019;20:e636–52. https://doi.org/10.1542/neo.20-11-e636.
Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984;259:14354–6.
Czerska M, Mikołajewska K, Zieliński M, Gromadzińska J, Wąsowicz W. Today's oxidative stress markers. Med Pr. 2015;66:393–405. https://doi.org/10.13075/mp.5893.00137.
Hu X, Tao C, Gan Q, Zheng J, Li H, You C. Oxidative stress in intracerebral hemorrhage: sources, mechanisms, and therapeutic targets. Oxid Med Cell Longev. 2016;2016:3215391. https://doi.org/10.1155/2016/3215391.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50. https://doi.org/10.1152/physrev.00026.2013.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001.
Martini S, Castellini L, Parladori R, Paoletti V, Aceti A, Corvaglia L. Free radicals and neonatal brain injury: from underlying pathophysiology to antioxidant treatment perspectives. Antioxidants (Basel). 2021;10 https://doi.org/10.3390/antiox10122012.
Orellana-Urzúa S, Claps G, Rodrigo R. Improvement of a novel proposal for antioxidant treatment against brain damage occurring in ischemic stroke patients. CNS Neurol Disord Drug Targets. 2021;20:3–21. https://doi.org/10.2174/1871527319666200910153431.
Dani C, Cecchi A, Bertini G. Role of oxidative stress as physiopathologic factor in the preterm infant. Minerva Pediatr. 2004;56:381–94.
Marseglia L, D'Angelo G, Manti S, Arrigo T, Barberi I, Reiter RJ, Gitto E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid Med Cell Longev. 2014;2014:358375. https://doi.org/10.1155/2014/358375.
Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid Med Cell Longev. 2016;2016:2768365. https://doi.org/10.1155/2016/2768365.
Dawes W. Secondary brain injury following neonatal intraventricular hemorrhage: the role of the ciliated ependyma. Front Pediatr. 2022;10:887606. https://doi.org/10.3389/fped.2022.887606.
Zhang Y, Khan S, Liu Y, Wu G, Yong VW, Xue M. Oxidative stress following intracerebral hemorrhage: from molecular mechanisms to therapeutic targets. Front Immunol. 2022;13:847246. https://doi.org/10.3389/fimmu.2022.847246.
Goulding DS, Vogel RC, Gensel JC, Morganti JM, Stromberg AJ, Miller BA. Acute brain inflammation, white matter oxidative stress, and myelin deficiency in a model of neonatal intraventricular hemorrhage. J Neurosurg Pediatr. 2020;26:613–23. https://doi.org/10.3171/2020.5.Peds20124.
Leijser LM, de Vries LS. Preterm brain injury: germinal matrix-intraventricular hemorrhage and post-hemorrhagic ventricular dilatation. Handb Clin Neurol. 2019;162:173–99. https://doi.org/10.1016/b978-0-444-64029-1.00008-4.
Niemczyk E, Majczak A, Hallmann A, Kedzior J, Woźniak M, Wakabayashi T. A possible involvement of plasma membrane NAD(P)H oxidase in the switch mechanism of the cell death mode from apoptosis to necrosis in menadione-induced cell injury. Acta Biochim Pol. 2004;51:1015–22.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. https://doi.org/10.1038/s41418-017-0012-4.
Li L, Yun D, Zhang Y, Tao Y, Tan Q, Qiao F, Luo B, Liu Y, Fan R, Xian J, et al. A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res. 2018;1697:113–23. https://doi.org/10.1016/j.brainres.2018.06.006.
Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316:C135–53. https://doi.org/10.1152/ajpcell.00136.2018.
Panfoli I, Candiano G, Malova M, De Angelis L, Cardiello V, Buonocore G, Ramenghi LA. Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front Pediatr. 2018;6:369. https://doi.org/10.3389/fped.2018.00369.
Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res. 1988;23:143–50. https://doi.org/10.1203/00006450-198802000-00001.
Liu Y, Wei J, Chang M, Liu Z, Li D, Hu S, Hu L. Proteomic analysis of endothelial progenitor cells exposed to oxidative stress. Int J Mol Med. 2013;32:607–14. https://doi.org/10.3892/ijmm.2013.1419.
Zia MT, Csiszar A, Labinskyy N, Hu F, Vinukonda G, LaGamma EF, Ungvari Z, Ballabh P. Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: role of NAD(P)H oxidase. Stroke. 2009;40:2191–8. https://doi.org/10.1161/strokeaha.108.544759.
Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–22. https://doi.org/10.1074/jbc.M209124200.
Kupsco A, Schlenk D. Oxidative stress, unfolded protein response, and apoptosis in developmental toxicity. Int Rev Cell Mol Biol. 2015;317:1–66. https://doi.org/10.1016/bs.ircmb.2015.02.002.
Yao Z, Bai Q, Wang G. Mechanisms of oxidative stress and therapeutic targets following intracerebral hemorrhage. Oxid Med Cell Longev. 2021;2021:8815441. https://doi.org/10.1155/2021/8815441.
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–19. https://doi.org/10.1002/ana.24070.
Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–9. https://doi.org/10.1016/s0166-2236(96)10074-6.
Goulay R, Naveau M, Gaberel T, Vivien D, Parcq J. Optimized tPA: a non-neurotoxic fibrinolytic agent for the drainage of intracerebral hemorrhages. J Cereb Blood Flow Metab. 2018;38:1180–9. https://doi.org/10.1177/0271678x17719180.
Wang Z, Chen Z, Yang J, Yang Z, Yin J, Duan X, Shen H, Li H, Wang Z, Chen G. Treatment of secondary brain injury by perturbing postsynaptic density protein-95-NMDA receptor interaction after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2019;39:1588–601. https://doi.org/10.1177/0271678x18762637.
Qu J, Chen W, Hu R, Feng H. The injury and therapy of reactive oxygen species in intracerebral hemorrhage looking at mitochondria. Oxid Med Cell Longev. 2016;2016:2592935. https://doi.org/10.1155/2016/2592935.
Hanafy KA, Gomes JA, Selim M. Rationale and current evidence for testing iron chelators for treating stroke. Curr Cardiol Rep. 2019;21:20. https://doi.org/10.1007/s11886-019-1106-z.
Li J, Cao F, Yin H-L, Huang Z-J, Lin Z-T, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death & Dis. 2020;11:88. https://doi.org/10.1038/s41419-020-2298-2.
Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–76. https://doi.org/10.1016/j.tcb.2015.10.014.
Wan J, Ren H, Wang J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol. 2019;4:93. https://doi.org/10.1136/svn-2018-000205.
Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. https://doi.org/10.1001/jamapediatrics.2014.3269.
Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed. 2017;102:F346–58. https://doi.org/10.1136/archdischild-2015-309639.
Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, Cooper CE, Wyatt JS, Reynolds EO, Mehmet H. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995;217:1193–9. https://doi.org/10.1006/bbrc.1995.2895.
Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–70. https://doi.org/10.1203/00006450-199505000-00019.
Schumacker PT, Rowland J, Saltz S, Nelson DP, Wood LD. Effects of hyperthermia and hypothermia on oxygen extraction by tissues during hypovolemia. J Appl Physiol. 1987;63:1246–52. https://doi.org/10.1152/jappl.1987.63.3.1246.
Thoresen M, Wyatt J. Keeping a cool head, post-hypoxic hypothermia--an old idea revisited. Acta Paediatr. 1997;86:1029–33. https://doi.org/10.1111/j.1651-2227.1997.tb14799.x.
Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444–58. https://doi.org/10.1007/bf02253360.
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res. 2018;9:74–91. https://doi.org/10.1007/s12975-017-0559-x.
Martini S, Austin T, Aceti A, Faldella G, Corvaglia L. Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives. Pediatr Res. 2020;87:823–33. https://doi.org/10.1038/s41390-019-0639-6.
Blanco S, Hernández R, Franchelli G, Ramos-Álvarez MM, Peinado M. Melatonin influences NO/NOS pathway and reduces oxidative and nitrosative stress in a model of hypoxic-ischemic brain damage. Nitric Oxide. 2017;62:32–43. https://doi.org/10.1016/j.niox.2016.12.001.
Matsuki N, Takanohashi A, Boffi FM, Inanami O, Kuwabara M, Ono K. Hydroxyl radical generation and lipid peroxidation in C2C12 myotube treated with iodoacetate and cyanide. Free Radic Res. 1999;31:1–8. https://doi.org/10.1080/10715769900300551.
Meng H, Li F, Hu R, Yuan Y, Gong G, Hu S, Feng H. Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res. 2015;1602:44–52. https://doi.org/10.1016/j.brainres.2014.08.039.
Cisternas P, Vio CP, Inestrosa NC. Role of Wnt signaling in tissue fibrosis, lessons from skeletal muscle and kidney. Curr Mol Med. 2014;14:510–22. https://doi.org/10.2174/1566524014666140414210346.
Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326–35. https://doi.org/10.1016/j.biochi.2013.09.003.
Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, Rolland W, Zhang JH. Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke. 2014;45:2475–9. https://doi.org/10.1161/strokeaha.114.005079.
Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, Zhu G, Feng H, Chen Z. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res. 2015;1594:115–24. https://doi.org/10.1016/j.brainres.2014.10.046.
Liu Y, Li Z, Khan S, Zhang R, Wei R, Zhang Y, Xue M, Yong VW. Neuroprotection of minocycline by inhibition of extracellular matrix metalloproteinase inducer expression following intracerebral hemorrhage in mice. Neurosci Lett. 2021;764:136297. https://doi.org/10.1016/j.neulet.2021.136297.
Li Z, Liu Y, Wei R, Khan S, Xue M, Yong VW. The combination of deferoxamine and minocycline strengthens neuroprotective effect on acute intracerebral hemorrhage in rats. Neurol Res. 2021;43:854–64. https://doi.org/10.1080/01616412.2021.1939487.
Flores JJ, Klebe D, Rolland WB, Lekic T, Krafft PR, Zhang JH. PPARγ-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol Dis. 2016;87:124–33. https://doi.org/10.1016/j.nbd.2015.12.015.
Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–62. https://doi.org/10.1002/ana.21097.
Chen Q, Shi X, Tan Q, Feng Z, Wang Y, Yuan Q, Tao Y, Zhang J, Tan L, Zhu G, et al. Simvastatin promotes hematoma absorption and reduces hydrocephalus following intraventricular hemorrhage in part by upregulating CD36. Transl Stroke Res. 2017;8:362–73. https://doi.org/10.1007/s12975-017-0521-y.
Chen Z, Zhang J, Chen Q, Guo J, Zhu G, Feng H. Neuroprotective effects of edaravone after intraventricular hemorrhage in rats. Neuroreport. 2014;25:635–40. https://doi.org/10.1097/wnr.0000000000000050.
Lekic T, Manaenko A, Rolland W, Fathali N, Peterson M, Tang J, Zhang JH. Protective effect of hydrogen gas therapy after germinal matrix hemorrhage in neonatal rats. Acta Neurochir Suppl. 2011;111:237–41. https://doi.org/10.1007/978-3-7091-0693-8_40.
Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J. Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:179–83. https://doi.org/10.1007/978-3-7091-0693-8_30.
Cheng B, Ballabh P. Recovery of the brain after intraventricular hemorrhage. Semin Fetal Neonatal Med. 2022;27:101224. https://doi.org/10.1016/j.siny.2021.101224.
Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, Ortega JA, Kayton R, Ungvari Z, Mongin AA, et al. AMPA-Kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J Neurosci. 2016;36:3363–77. https://doi.org/10.1523/jneurosci.4329-15.2016.
Goddard J, Lewis RM, Alcala H, Zeller RS. Intraventricular hemorrhage--an animal model. Biol Neonate. 1980;37:39–52. https://doi.org/10.1159/000241254.
Wheeler AS, Sadri S, Gutsche BB, DeVore JS, David-Mian Z, Latyshevsky H. Intracranial hemorrhage following intravenous administration of sodium bicarbonate or saline solution in the newborn lamb asphyxiated in utero. Anesthesiology. 1979;51:517–21. https://doi.org/10.1097/00000542-197912000-00007.
Reynolds ML, Evans CA, Reynolds EO, Saunders NR, Durbin GM, Wigglesworth JS. Intracranial haemorrhage in the preterm sheep fetus. Early Hum Dev. 1979;3:163–86. https://doi.org/10.1016/0378-3782(79)90005-7.
Lekic T, Manaenko A, Rolland W, Tang J, Zhang JH. A novel preclinical model of germinal matrix hemorrhage using neonatal rats. Acta Neurochir Suppl. 2011;111:55–60. https://doi.org/10.1007/978-3-7091-0693-8_10.
Segado-Arenas A, Infante-Garcia C, Benavente-Fernandez I, Sanchez-Sotano D, Ramos-Rodriguez JJ, Alonso-Ojembarrena A, Lubian-Lopez S, Garcia-Alloza M. Cognitive impairment and brain and peripheral alterations in a murine model of intraventricular hemorrhage in the preterm newborn. Mol Neurobiol. 2018;55:4896–910. https://doi.org/10.1007/s12035-017-0693-1.
Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Küker W, Gilsbach JM. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–8. https://doi.org/10.1161/01.str.28.1.141.
Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–33. https://doi.org/10.1016/j.cell.2004.12.020.
Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. https://doi.org/10.1016/j.mcn.2005.02.006.
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001.
Atienza-Navarro I, Alves-Martinez P, Lubian-Lopez S, Garcia-Alloza M. Germinal matrix-intraventricular hemorrhage of the preterm newborn and preclinical models: inflammatory considerations. Int J Mol Sci. 2020;21 https://doi.org/10.3390/ijms21218343.
McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–77. https://doi.org/10.1128/mcb.22.21.7667-7677.2002.
Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71. https://doi.org/10.1126/science.1109418.
Yang D, Baumann JM, Sun YY, Tang M, Dunn RS, Akeson AL, Kernie SG, Kallapur S, Lindquist DM, Huang EJ, et al. Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci Transl Med. 2013;5:193ra190. https://doi.org/10.1126/scitranslmed.3005794.
Dohare P, Cheng B, Ahmed E, Yadala V, Singla P, Thomas S, Kayton R, Ungvari Z, Ballabh P. Glycogen synthase kinase-3β inhibition enhances myelination in preterm newborns with intraventricular hemorrhage, but not recombinant Wnt3A. Neurobiol Dis. 2018;118:22–39. https://doi.org/10.1016/j.nbd.2018.06.015.
Fischer EG, Lorenzo AV, Landis WJ, Welch K, Ofori-Kwakye SK, Dorval B, Hodgens KJ, Kerr CS. Vasculature to the germinal matrix in rabbit pups. J Neurosurg. 1986;64:650–6. https://doi.org/10.3171/jns.1986.64.4.0650.
Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39:3378–88. https://doi.org/10.1161/strokeaha.107.510883.
Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AG, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. J Neuropathol Exp Neurol. 2003;62:292–303. https://doi.org/10.1093/jnen/62.3.292.
Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 2014;75:696–705; discussion 706,. https://doi.org/10.1227/neu.0000000000000524.
Li P, Zhao G, Ding Y, Wang T, Flores J, Ocak U, Wu P, Zhang T, Mo J, Zhang JH, et al. Rh-IFN-α attenuates neuroinflammation and improves neurological function by inhibiting NF-κB through JAK1-STAT1/TRAF3 pathway in an experimental GMH rat model. Brain Behav Immun. 2019;79:174–85. https://doi.org/10.1016/j.bbi.2019.01.028.
Tao Y, Li L, Jiang B, Feng Z, Yang L, Tang J, Chen Q, Zhang J, Tan Q, Feng H, et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav Immun. 2016;58:118–29. https://doi.org/10.1016/j.bbi.2016.05.020.
Lorenzo AV, Welch K. Preterm rabbit model of intraventricular hemorrhage. J Neurosurg. 1986;64:688–9. https://doi.org/10.3171/jns.1986.64.4.0688.
Lorenzo AV, Welch K, Conner S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. J Neurosurg. 1982;56:404–10. https://doi.org/10.3171/jns.1982.56.3.0404.
Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–6. https://doi.org/10.1161/strokeaha.110.596718.
Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–6. https://doi.org/10.1038/nature10487.
Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–6. https://doi.org/10.1038/nn.3604.
Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, Greenberg SM, James ML, Singh V, Clark WM, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18:428–38. https://doi.org/10.1016/s1474-4422(19)30069-9.
Wei C, Wang J, Foster LD, Yeatts SD, Moy C, Mocco J, Selim M, Palesch Y, Griffin J, Perlmutter A, et al. Effect of deferoxamine on outcome according to baseline hematoma volume: a post hoc analysis of the i-DEF trial. Stroke. 2022;53:1149–56. https://doi.org/10.1161/STROKEAHA.121.035421.
Ohlsson A, Roberts RS, Schmidt B, Davis P, Moddeman D, Saigal S, Solimano A, Vincer M, Wright L. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147:860–2. https://doi.org/10.1016/j.jpeds.2005.07.032.
Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–6. https://doi.org/10.1542/peds.111.4.e340.
Dietz RM, Deng G, Orfila JE, Hui X, Traystman RJ, Herson PS. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience. 2016;325:132–41. https://doi.org/10.1016/j.neuroscience.2016.03.052.
D'Angelo G, Chimenz R, Reiter RJ, Gitto E. Use of melatonin in oxidative stress related neonatal diseases. Antioxidants (Basel). 2020;9 https://doi.org/10.3390/antiox9060477.
Elser HE, Holditch-Davis D, Brandon DH. Cerebral oxygenation monitoring: a strategy to detect IVH and PVL. Newborn Infant Nurs Rev. 2011;11:153–9. https://doi.org/10.1053/j.nainr.2011.07.007.
Variane GFT, Chock VY, Netto A, Pietrobom RFR, Van Meurs KP. Simultaneous near-infrared spectroscopy (NIRS) and amplitude-integrated electroencephalography (aEEG): dual use of brain monitoring techniques improves our understanding of physiology. Front Pediatr. 2020;7 https://doi.org/10.3389/fped.2019.00560.
Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. https://doi.org/10.1002/14651858.CD004454.pub3.
Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009:CD004661. https://doi.org/10.1002/14651858.CD004661.pub3.
Szymonowicz W, Yu VY, Walker A, Wilson F. Reduction in periventricular haemorrhage in preterm infants. Arch Dis Child. 1986;61:661–5. https://doi.org/10.1136/adc.61.7.661.
de Bijl-Marcus K, Brouwer AJ, De Vries LS, Groenendaal F, Wezel-Meijler GV. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: a multicentre cohort study. Arch Dis Child Fetal Neonatal Ed. 2020;105:419–24. https://doi.org/10.1136/archdischild-2018-316692.
Garvey AA, Kooi EMW, Smith A, Dempsey EM. Interpretation of cerebral oxygenation changes in the preterm infant. Children (Basel). 2018;5 https://doi.org/10.3390/children5070094.
Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82:F188–94. https://doi.org/10.1136/fn.82.3.f188.
Sortica da Costa C, Cardim D, Molnar Z, Kelsall W, Ng I, Czosnyka M, Smielewski P, Austin T. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr Res. 2019;86:247–53. https://doi.org/10.1038/s41390-019-0410-z.
Hansen ML, Pellicer A, Gluud C, Dempsey E, Mintzer J, Hyttel-Sørensen S, Heuchan AM, Hagmann C, Ergenekon E, Dimitriou G, et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the SafeBoosC randomised clinical phase III trial. Trials. 2019;20:811. https://doi.org/10.1186/s13063-019-3955-6.
Funding
No funding or financial support was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nour Eldine, M., Alhousseini, M., Nour-Eldine, W. et al. The Role of Oxidative Stress in the Progression of Secondary Brain Injury Following Germinal Matrix Hemorrhage. Transl. Stroke Res. 15, 647–658 (2024). https://doi.org/10.1007/s12975-023-01147-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-023-01147-3