Abstract
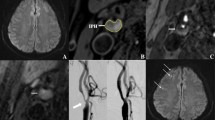
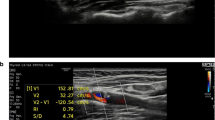
A significant portion of ischemic stroke is on account of emboli caused by fibrous cap rupture of vulnerable plaque with intraplaque neovascularization as a significant triggering factor to plaque vulnerability. Contrast-enhanced ultrasound (CEUS) could offer detailed information on plaque surface and intraplaque microvascular. This study aims to comprehensively assess the value of CEUS for the detection of plaque rupture and neovascularization in histologically verified plaques that had been removed from the patients who had undergone carotid endarterectomy (CEA). Fifty-one consecutive subjects (mean age, 67.0 ± 6.5 years; 43 [84.3%] men) scheduled for CEA were recruited. Standard ultrasound and CEUS were performed prior to surgery. Based on the direction of the contrast agents that diffuse within the plaques, plaques were divided as “inside-out” direction (contrast agents diffuse from the artery lumen towards the inside of the plaque) and non-inside-out direction. Plaque enhancement was assessed by using a semi-quantitative grading scale (grade 1: no enhancement; grade 2: moderate enhancement; grade 3: extensive enhancement). Plaques were evaluated for histopathologic characteristics according to Oxford Plaque Study (OPS) standard postoperative. Intraplaque neovascularization as manifested by the appearance of CD34-positive microvessels was characterized in terms of microvessel density (MVD), microvessel area (MVA), and microvessel shape (MVS). In 51 plaques, the sensitivity, specificity, positive, and negative predictive values of contrast agent inside-out direction diffusion for the detection of plaque fibrous cap rupture were 87.5%, 92.6%, 91.3%, and 89.3%, respectively. The incidence of cap rupture was significantly higher in contrast agent inside-out direction diffusion than non-inside-out direction diffusion (73.9% vs 25.0%, p < 0.001), and inside-out direction diffusion did exhibit higher frequency of vulnerable plaques (OPS grades 3–4) (95.7% vs 53.6%, p = 0.001). Multivariate logistic regression analysis revealed the contrast agent inside-out direction diffusion as an independent correlate to plaque rupture (OR 8.5, 95% CI 2.4–30.1, p = 0.001). With increasing plaque enhancement, plaque MVD (p < 0.001), plaque MVA (p = 0.012), and percentage of highly irregular-shaped microvessels increased (p < 0.001). Contrast agent inside-out direction diffusion could indicate plaque rupture. The increase in plaque enhancement paralleled increased, larger, and more irregular-shaped microvessels, which may suggest an increased risk of plaque vulnerability.


Similar content being viewed by others
Abbreviations
- AHA:
-
American Heart Association
- BMI:
-
Body mass index
- CEUS:
-
Contrast-enhanced ultrasound
- FCR:
-
Fibrous cap rupture
- HDL-C:
-
High-density lipoprotein cholesterol
- HE:
-
Hematoxylin and eosin
- hs-CRP:
-
High-sensitive C-reactive protein
- IPH:
-
Intraplaque hemorrhage
- LDL-C:
-
Low-density lipoprotein cholesterol
- LLC:
-
Large lipid core
- MVA:
-
Microvessel area
- MVD:
-
Microvessel density
- MVS:
-
Microvessel shape
- NASCET:
-
North American Symptomatic Carotid Endarterectomy Trial
- OPS:
-
Oxford Plaque Study
- US:
-
Ultrasound
References
Howard DP, van Lammeren GW, Rothwell PM, et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46(1):182–9.
U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol. 2009;8(6):569–80.
Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2316–25.
Tanaka A, Imanishi T, Kitabata H, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118(23):2368–73.
Saba L, Saam T, Jager HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18(6):559–72.
Molloy KJ, Thompson MM, Jones JL, et al. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation. 2004;110(3):337–43.
Arbab-Zadeh A, Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(12):1582–93.
Stone GW, Mintz GS, Virmani R. Vulnerable plaques, vulnerable patients, and intravascular imaging. J Am Coll Cardiol. 2018;72(17):2022–6.
Ramanathan R, Dey D, Nørgaard BL, et al. Carotid plaque composition by CT angiography in asymptomatic subjects: a head-to-head comparison to ultrasound. Eur Radiol. 2019;29(11):5920–31.
ten KGL, van Dijk AC, van den Oord SC, et al. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol. 2013;112(2):292–8.
Feinstein SB, Coll B, Staub D, et al. Contrast enhanced ultrasound imaging. J Nucl Cardiol. 2010;17(1):106–15.
Vicenzini E, Giannoni MF, Puccinelli F, et al. Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke. 2007;38(10):2841–3.
D'Oria M, Chiarandini S, Pipitone MD, et al. Contrast enhanced ultrasound (CEUS) is not able to identify vulnerable plaques in asymptomatic carotid atherosclerotic disease. Eur J Vasc Endovasc Surg. 2018;56(5):632–42.
North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22(6):711–20.
Zamani M, Skagen K, Scott H, Lindberg B, Russell D, Skjelland M. Carotid plaque neovascularization detected with superb microvascular imaging ultrasound without using contrast media. Stroke. 2019;50(11):3121–7.
Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258(2):618–26.
van den Oord SC, Akkus Z, Renaud G, et al. Assessment of carotid atherosclerosis, intraplaque neovascularization, and plaque ulceration using quantitative contrast-enhanced ultrasound in asymptomatic patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2014;15(11):1213–8.
Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113(19):2320–8.
Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664–72.
Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20(5):1177–8.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
van den Oord SC, Akkus Z, van Lennep JER, et al. Assessment of subclinical atherosclerosis and intraplaque neovascularization using quantitative contrast-enhanced ultrasound in patients with familial hypercholesterolemia. Atherosclerosis. 2013;231(1):107–13.
Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218(1):7–29.
Brezinski M, Willard F, Rupnick M. Inadequate intimal angiogenesis as a source of coronary plaque instability: implications for healing. Circulation. 2019;140(23):1857–9.
Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52(3):223–30.
Mehta KS, Lee JJ, Taha AG, Avgerinos E, Chaer RA. Vascular applications of contrast-enhanced ultrasound imaging. J Vasc Surg. 2017;66(1):266–74.
Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53(17):1517–27.
Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45(1):155–9.
McCarthy MJ, Loftus IM, Thompson MM, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg. 1999;30(2):261–8.
Funding
This study was supported by Cadre Health Care Research Project of Jiangsu Province (No. BJ17010), People’s Livelihood Science and Technology Demonstration Project of Suzhou (No. SS201714, No. SS201859), and thirteen major projects of the Ministry of Science And Technology of China (No. 2017 YFC0114302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2019124).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lyu, Q., Tian, X., Ding, Y. et al. Evaluation of Carotid Plaque Rupture and Neovascularization by Contrast-Enhanced Ultrasound Imaging: an Exploratory Study Based on Histopathology. Transl. Stroke Res. 12, 49–56 (2021). https://doi.org/10.1007/s12975-020-00825-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00825-w