Abstract
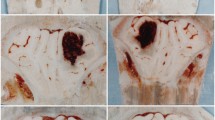
Intracerebral hemorrhage in combination with intraventricular hemorrhage (IVH) is a severe type of stroke frequently leading to prolonged clinical care, continuous disability, shunt dependency, and high mortality. The molecular mechanisms induced by IVH are complex and not fully understood. Moreover, the treatment options for IVH are limited. Intraventricular recombinant tissue plasminogen activator (rt-PA) dissolves the blood clot in the ventricular system; however, whether the clinical outcome is thereby positively affected is still being debated. The mechanistic cascade induced by intraventricular rt-PA therapy may cure and harm in parallel. Despite the fact that intraventricular blood clots are thereby dissolved, blood derivatives enter the parenchyma and may still adversely affect functional structures of the brain: Smaller blood clots may obstruct the perivascular (Virchow-Robin) space and thereby the glymphatic system with detrimental consequences for cerebrospinal fluid (CSF)/interstitial fluid (ISF) flow. These clots, blood cells but also blood derivatives in the perivascular space, destabilize the blood-brain barrier from the brain parenchyma side, thereby also functionally weakening the neurovascular unit. This may lead to further accommodation of serum proteins in the ISF and particularly in the perivascular space further contributing to the adverse effects on the neuronal microenvironment. Finally, the arterial (Pacchionian) granulations have to cope with ISF containing this “blood, cell, and protein cocktail,” resulting in obstruction and insufficient function of the arterial granulations, followed by a malresorptive hydrocephalus. Particularly in light of currently improved knowledge on the physiologic and pathophysiologic clearance of cerebrospinal fluid and interstitial fluid, a critical discussion and reevaluation of our current therapeutic strategies to treat intraventricular hemorrhages are needed to successfully treat patients suffering from this severe type of stroke. In this review, we therefore summarize and discuss recent clinical trials and future directions for the field of IVH with respect to the currently increased understanding of the glymphatic system and the neurovascular unit pathophysiology.


Similar content being viewed by others
References
Jaffe J, Melnychuk E, Muschelli J, Ziai W, Morgan T, Hanley DF, et al. Ventricular catheter location and the clearance of intraventricular hemorrhage. Neurosurgery. 2012;70(5):1258–63; discussion 63-4. https://doi.org/10.1227/NEU.0b013e31823f6571.
Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. 2013;80(14):1295–9. https://doi.org/10.1212/WNL.0b013e31828ab2a7.
Nowinski WL, Gomolka RS, Qian G, Gupta V, Ullman NL, Hanley DF. Characterization of intraventricular and intracerebral hematomas in non-contrast CT. Neuroradiol J. 2014;27(3):299–315. https://doi.org/10.15274/NRJ-2014-10042.
Rabinstein AA, Anderson CD. Time is brain also counts for ICH. Neurology. 2015;84(10):970–1. https://doi.org/10.1212/WNL.0000000000001349.
Witsch J, Bruce E, Meyers E, Velazquez A, Schmidt JM, Suwatcharangkoon S, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84(10):989–94. https://doi.org/10.1212/WNL.0000000000001344.
Ziai WC, Nyquist PA, Hanley DF. Ventriculostomy and lytic therapy for intracerebral hemorrhage. Front Neurol Neurosci. 2015;37:130–47. https://doi.org/10.1159/000437118.
Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke. 2014;9(4):536–42. https://doi.org/10.1111/ijs.12097.
Herrick DB, Ziai WC, Thompson CB, Lane K, McBee NA, Hanley DF. Systemic hematologic status following intraventricular recombinant tissue-type plasminogen activator for intraventricular hemorrhage: the CLEAR IVH Study Group. Stroke. 2011;42(12):3631–3. https://doi.org/10.1161/STROKEAHA.111.625749.
Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42(11):3009–16. https://doi.org/10.1161/STROKEAHA.110.610949.
Webb AJ, Ullman NL, Mann S, Muschelli J, Awad IA, Hanley DF. Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: the Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR IVH) program. Stroke. 2012;43(6):1666–8. https://doi.org/10.1161/STROKEAHA.112.650523.
Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. https://doi.org/10.1016/S0140-6736(16)32410-2.
Fam MD, Pang A, Zeineddine HA, Mayo S, Stadnik A, Jesselson M, et al. Demographic risk factors for vascular lesions as etiology of intraventricular hemorrhage in prospectively screened cases. Cerebrovasc Dis. 2017;43(5–6):223–30. https://doi.org/10.1159/000458452.
Fam MD, Zeineddine HA, Eliyas JK, Stadnik A, Jesselson M, McBee N, et al. CSF inflammatory response after intraventricular hemorrhage. Neurology. 2017;89(15):1553–60. https://doi.org/10.1212/WNL.0000000000004493.
Murthy SB, Awad I, Harnof S, Aldrich F, Harrigan M, Jallo J, et al. Permanent CSF shunting after intraventricular hemorrhage in the CLEAR III trial. Neurology. 2017;89(4):355–62. https://doi.org/10.1212/WNL.0000000000004155.
Fam MD, Hanley D, Stadnik A, Zeineddine HA, Girard R, Jesselson M, et al. Surgical performance in minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation phase III clinical trial. Neurosurgery. 2017;81(5):860–6. https://doi.org/10.1093/neuros/nyx123.
Oreskovic D, Rados M, Klarica M. Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience. 2017;354:69–87. https://doi.org/10.1016/j.neuroscience.2017.04.025.
Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci. 2017;37(9):2395–402. https://doi.org/10.1523/JNEUROSCI.2754-16.2017.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11. https://doi.org/10.1126/scitranslmed.3003748.
Bosche B, Graf R, Ernestus RI, Dohmen C, Reithmeier T, Brinker G, et al. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol. 2010;67(5):607–17. https://doi.org/10.1002/ana.21943.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. https://doi.org/10.1016/j.neuron.2017.07.030.
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45(10):3092–6. https://doi.org/10.1161/STROKEAHA.114.006617.
Fisher MJ. Brain regulation of thrombosis and hemostasis: from theory to practice. Stroke. 2013;44(11):3275–85. https://doi.org/10.1161/STROKEAHA.113.000736.
Bosche B, Molcanyi M, Noll T, Kochanek M, Kraus B, Rieger B, et al. Occurrence and recurrence of spontaneous chronic subdural haematoma is associated with a factor XIII deficiency. Clin Neurol Neurosurg. 2013;115(1):13–8. https://doi.org/10.1016/j.clineuro.2012.03.045.
Burggraf D, Martens HK, Dichgans M, Hamann GF. rt-PA causes a dose-dependent increase in the extravasation of cellular and non-cellular blood elements after focal cerebral ischemia. Brain Res. 2007;1164:55–62. https://doi.org/10.1016/j.brainres.2007.05.066.
Renu A, Laredo C, Lopez-Rueda A, Llull L, Tudela R, San-Roman L, et al. Vessel wall enhancement and blood-cerebrospinal fluid barrier disruption after mechanical Thrombectomy in acute ischemic stroke. Stroke. 2017;48(3):651–7. https://doi.org/10.1161/STROKEAHA.116.015648.
Vivien D. Can the benefits of rtPA treatment for stroke be improved? Rev Neurol (Paris). 2017;173(9):566–71. https://doi.org/10.1016/j.neurol.2017.07.003.
Staykov D, Kuramatsu JB, Bardutzky J, Volbers B, Gerner ST, Kloska SP, et al. Efficacy and safety of combined intraventricular fibrinolysis with lumbar drainage for prevention of permanent shunt dependency after intracerebral hemorrhage with severe ventricular involvement: a randomized trial and individual patient data meta-analysis. Ann Neurol. 2017;81(1):93–103. https://doi.org/10.1002/ana.24834.
Gilard V, Djoubairou BO, Lepetit A, Metayer T, Gakuba C, Gourio C, et al. Small versus large catheters for ventriculostomy in the management of intraventricular hemorrhage. World Neurosurg. 2017;97:117–22. https://doi.org/10.1016/j.wneu.2016.09.105.
Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology. 2017;127(6):976–88. https://doi.org/10.1097/ALN.0000000000001888.
Bosche B, Molcanyi M, Noll T, Rej S, Zatschler B, Doeppner TR, et al. A differential impact of lithium on endothelium-dependent but not on endothelium-independent vessel relaxation. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;67:98–106. https://doi.org/10.1016/j.pnpbp.2016.02.004.
Bosche B, Molcanyi M, Rej S, Doeppner TR, Obermann M, Muller DJ, et al. Low-dose lithium stabilizes human endothelial barrier by decreasing MLC phosphorylation and universally augments cholinergic vasorelaxation capacity in a direct manner. Front Physiol. 2016;7:593. https://doi.org/10.3389/fphys.2016.00593.
Bosche B, Schafer M, Graf R, Hartel FV, Schafer U, Noll T. Lithium prevents early cytosolic calcium increase and secondary injurious calcium overload in glycolytically inhibited endothelial cells. Biochem Biophys Res Commun. 2013;434(2):268–72. https://doi.org/10.1016/j.bbrc.2013.03.047.
Bedussi B, van der Wel NN, de Vos J, van Veen H, Siebes M, VanBavel E, et al. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: a single compartment with preferential pathways. J Cereb Blood Flow Metab. 2017;37(4):1374–85. https://doi.org/10.1177/0271678X16655550.
Faghih MM, Sharp MK. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS. 2018;15(1):17. https://doi.org/10.1186/s12987-018-0103-8.
Staykov D, Huttner HB, Struffert T, Ganslandt O, Doerfler A, Schwab S, et al. Intraventricular fibrinolysis and lumbar drainage for ventricular hemorrhage. Stroke. 2009 Oct;40(10):3275–80. https://doi.org/10.1161/STROKEAHA.109.551945.
Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21(12):1111–22. https://doi.org/10.1080/14728222.2017.1397628.
Abdelmalik PA, Ziai WC. Spontaneous intraventricular hemorrhage: when should intraventricular tPA be considered? Semin Respir Crit Care Med. 2017;38(6):745–59. https://doi.org/10.1055/s-0037-1607991.
Acknowledgments
The authors acknowledge Sebastian Zachar, M.F.A. for creating the illustrations of Fig. 2 based on the drafts by BB and MM.
Funding
BB was supported by grants of the Deutsche Forschungsgemeinschaft (BO 4229/1-1, BO 4229/2-1, novel strategies to protect the neurovascular unit in cerebrovascular diseases) and PM by the European Union’s Seventh Framework Program (FP7/2008–2013) under grant agreement 627951 (Marie Curie IOF), the German Academic Exchange Service DAAD PPP Canada program with funds of the German Federal Ministry of Education and Research (BMBF, grant no. 57212163), and the German Federal Ministry of Education and Research (BMBF, grant no. 16GW0191). PM is a fellow of the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Author information
Authors and Affiliations
Contributions
BB wrote the initial draft of the manuscript, which was intellectually extended by PM and MM, and finally re-edited by BB, PM, TD, JH, and MM. BB, TD, and MM created the drafts of the figures. All authors substantially contributed to the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Bosche, B., Mergenthaler, P., Doeppner, T.R. et al. Complex Clearance Mechanisms After Intraventricular Hemorrhage and rt-PA Treatment—a Review on Clinical Trials. Transl. Stroke Res. 11, 337–344 (2020). https://doi.org/10.1007/s12975-019-00735-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00735-6